Abstract
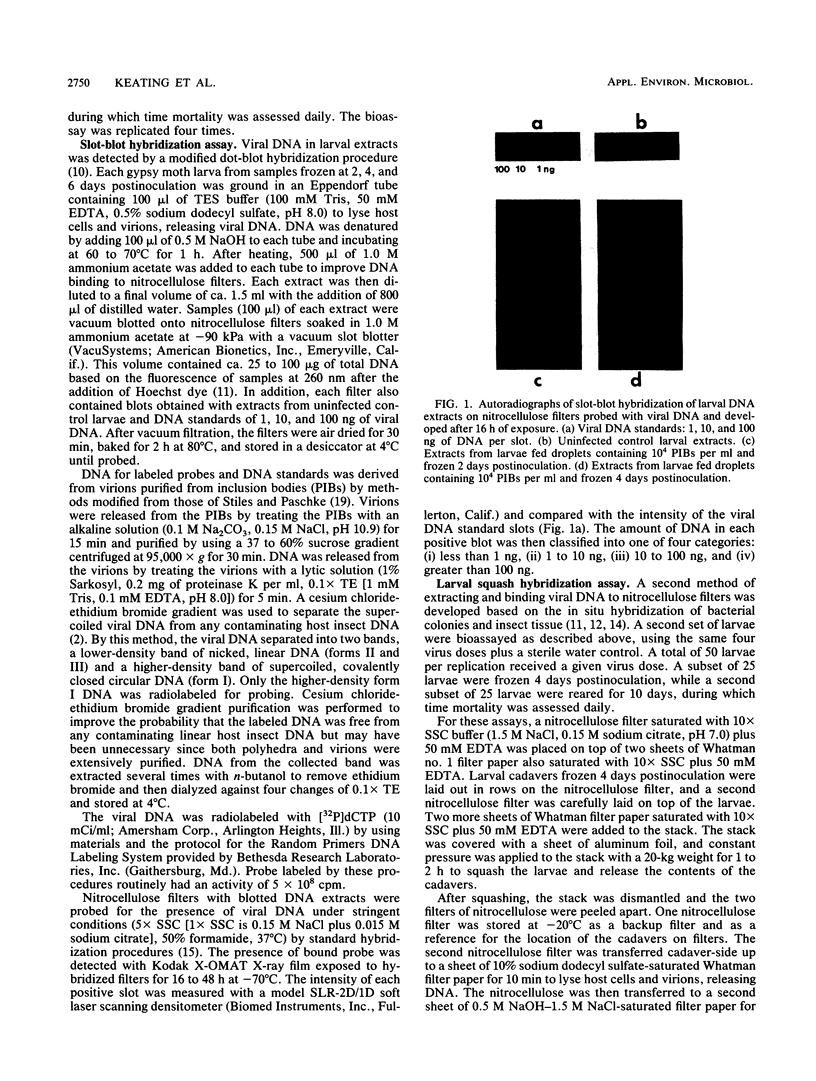
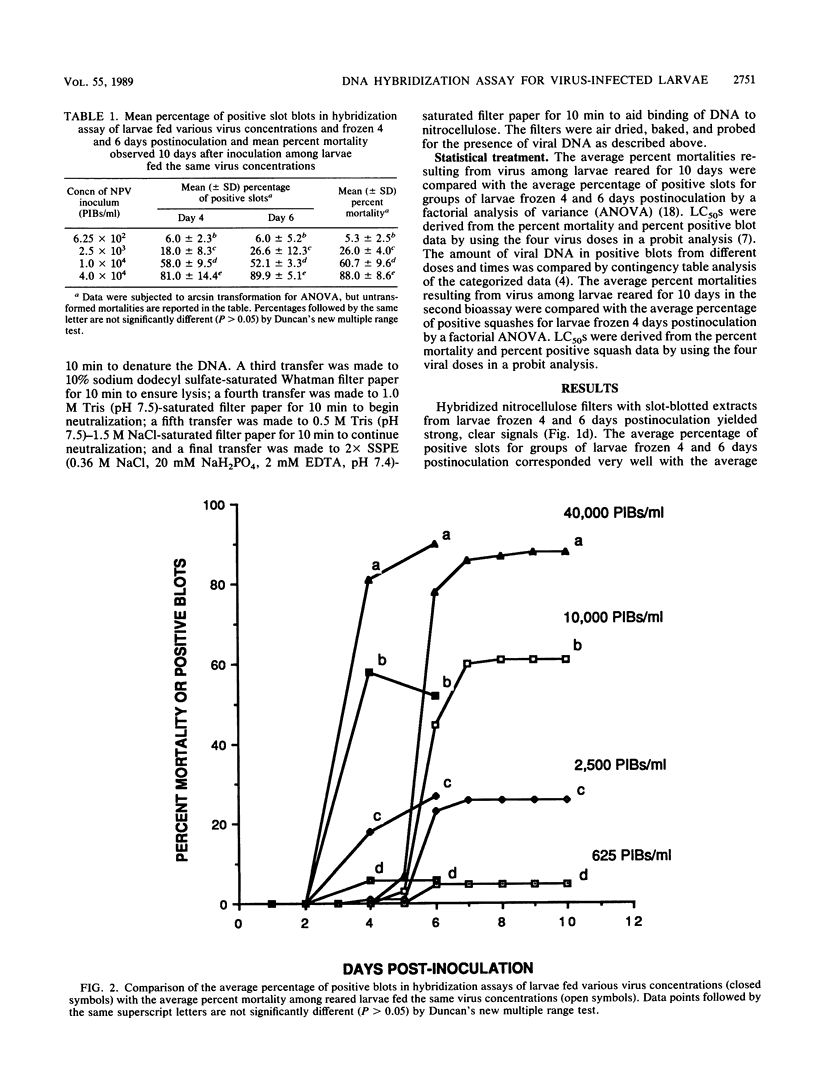
Radiolabeled Lymantria dispar nuclear polyhedrosis virus DNA probes were used in a DNA hybridization assay to detect the presence of viral DNA in extracts from infected larvae. Total DNA was extracted from larvae, bound to nitrocellulose filters, and assayed for the presence of viral DNA by two methods: slot-blot vacuum filtration and whole-larval squashes. To test the assays, neonate larvae were fed droplets containing a known concentration of L. dispar nuclear polyhedrosis virus and observed for up to 10 days to determine the percentage of infected larvae. The average percent mortalities were 88.0, 60.7, 26.0, and 5.3% for larvae fed droplets containing 4.0 x 10(4), 1.0 x 10(4), 2.5 x 10(3), and 6.25 x 10(2) polyhedral inclusion bodies (PIBs) per ml, respectively. Other larvae treated with the same virus concentrations were frozen at 2, 4, and 6 days postinoculation and examined by the hybridization techniques. The average percentage of slot blots containing viral DNA equaled 81.0, 58.0, 18.0, and 6.0% for larvae blotted 4 days after treatment with 4.0 x 10(4), 1.0 x 10(4), 2.5 x 10(3), and 6.25 x 10(2) PIBs per ml, respectively, and 89.9, 52.1, 26.6, and 6.0%, respectively at 6 days postinoculation. Thus, the hybridization results were closely correlated with mortality observed in reared larvae. Hybridization of squashes of larvae frozen 4 days after receiving the above virus treatments also produced accurate measures of the incidence of virus infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell R. W., Podgwaite J. D. The disease complex of the gypsy moth. I. Major components. J Invertebr Pathol. 1971 Jul;18(1):101–107. doi: 10.1016/0022-2011(91)90015-i. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Le Parco Y., Cecchini J. P., Knibiehler B., Mirre C. Characterization and expression of collagen-like genes in Drosophila melanogaster. Biol Cell. 1986;56(3):217–226. doi: 10.1111/j.1768-322x.1986.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Smith I. R., van Beek N. A., Podgwaite J. D., Wood H. A. Physical map and polyhedrin gene sequence of Lymantria dispar nuclear polyhedrosis virus. Gene. 1988 Nov 15;71(1):97–105. doi: 10.1016/0378-1119(88)90081-9. [DOI] [PubMed] [Google Scholar]