Abstract
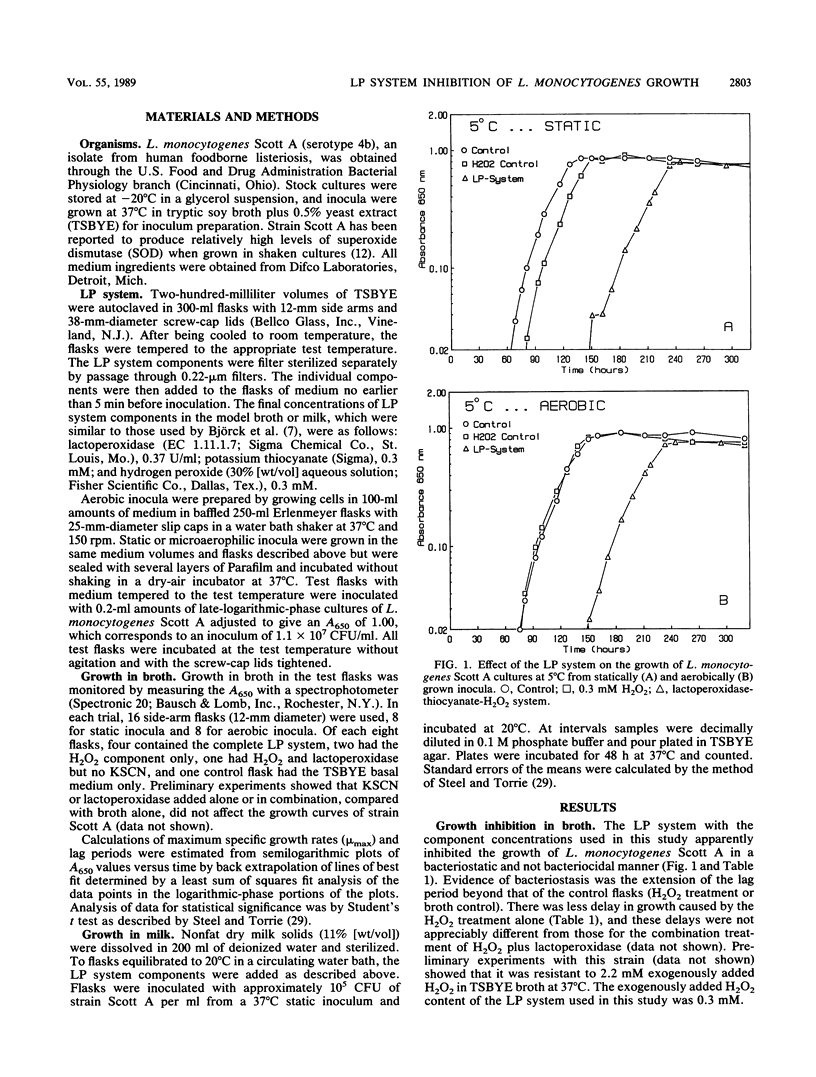
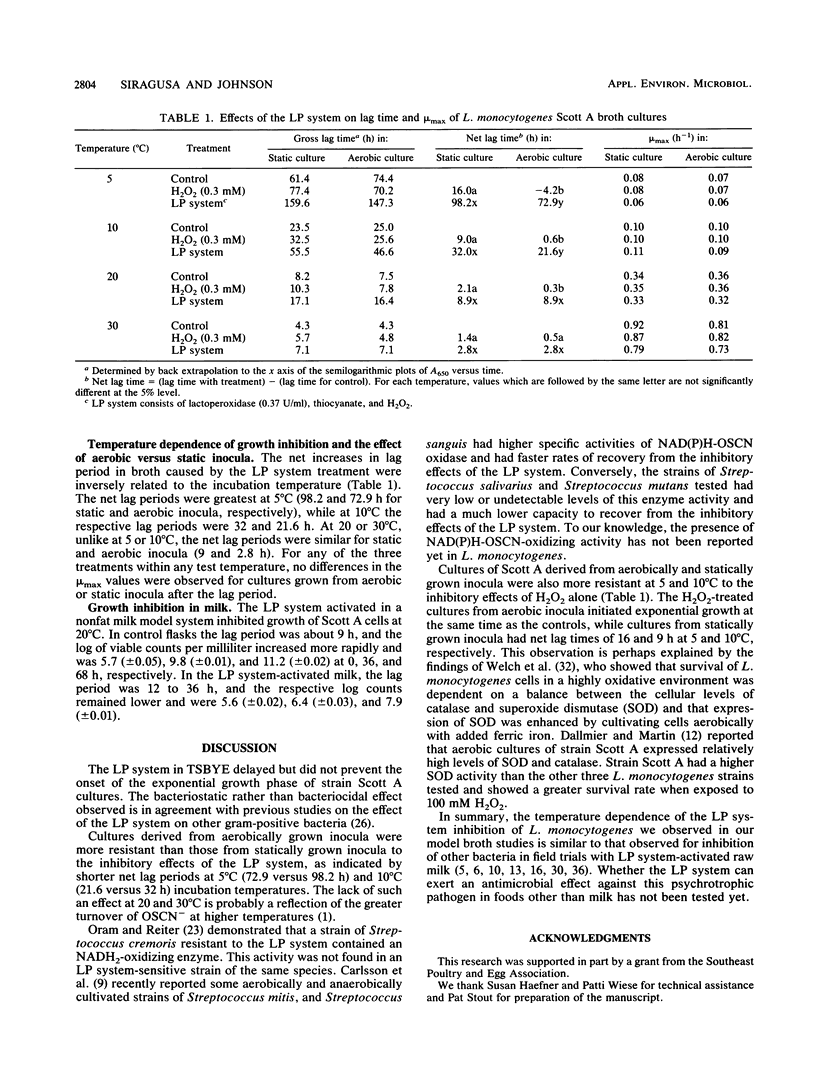
The lactoperoxidase-thiocyanate-H2O2 system (LP system), consisting of lactoperoxidase (0.37 U/ml), KSCN (0.3 mM), and H2O2 (0.3 mM), delayed but did not prevent growth of L. monocytogenes Scott A at 5, 10, 20, and 30 degrees C in broth and at 20 degrees C in milk. The net lag periods determined spectrophotometrically varied inversely with temperature and were shorter at 5 and 10 degrees C for cultures from shaken versus from statically grown inocula. Lag periods for cultures from shaken and statically grown inocula, respectively, were 73 and 98 h at 5 degrees C, 22 and 32 h at 10 degrees C, both 8.9 h at 20 degrees C, and both 2.8 h at 30 degrees C. After the lag periods, the maximum specific growth rates were similar for each of the three treatments (complete LP system, H2O2 alone, or control broth) at 5, 10, and 20 degrees C and were 0.06 to 0.08, 0.09 to 0.1, and 0.32 to 0.36/h, respectively. At 20 degrees C in sterile reconstituted skim milk, the LP system restricted growth of Scott A, with log CFU counts per ml at 0, 36, and 68 h being 5.7, 6.4 and 7.9 (versus 5.7, 9.8, and 11.2 for controls). Possible explanations for the decreased lag times observed for cultures from aerobically grown inocula are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Björck L. Antibacterial effect of the lactoperoxidase system on psychotrophic bacteria in milk. J Dairy Res. 1978 Feb;45(1):109–118. doi: 10.1017/s0022029900016253. [DOI] [PubMed] [Google Scholar]
- Björck L., Rosén C., Marshall V., Reiter B. Antibacterial activity of the lactoperoxidase system in milk against pseudomonads and other gram-negative bacteria. Appl Microbiol. 1975 Aug;30(2):199–204. doi: 10.1128/am.30.2.199-204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R., Vandenbroucke-Grauls C. M., van Asbeck B. S., Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987 Dec;55(12):3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol. 1989 Apr;171(4):2188–2194. doi: 10.1128/jb.171.4.2188-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988 Feb;54(2):581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P. S., Feeley J. C., Graves L. M., Ajello G. W., Fleming D. W. Isolation of Listeria monocytogenes from raw milk. Appl Environ Microbiol. 1986 Feb;51(2):438–440. doi: 10.1128/aem.51.2.438-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnan M. J., Mascola L., Lou X. D., Goulet V., May S., Salminen C., Hird D. W., Yonekura M. L., Hayes P., Weaver R. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988 Sep 29;319(13):823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- Marshall V. M., Reiter B. Comparison of the antibacterial activity of the hypothiocyanite anion towards Streptococcus lactis and Escherichia coli. J Gen Microbiol. 1980 Oct;120(2):513–516. doi: 10.1099/00221287-120-2-513. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Antibacterial action of lactoperoxidase-thiocyanate-hydrogen peroxide on Streptococcus agalactiae. Appl Environ Microbiol. 1979 Nov;38(5):821–826. doi: 10.1128/aem.38.5.821-826.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy M. A., Tenovuo J., Pruitt K. M., White W. E., Jr Effect of growth phase and cell envelope structure on susceptibility of Salmonella typhimurium to the lactoperoxidase-thiocyanate-hydrogen peroxide system. Infect Immun. 1983 Mar;39(3):1187–1195. doi: 10.1128/iai.39.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B., Marshall V. M., BjörckL, Rosén C. G. Nonspecific bactericidal activity of the lactoperoxidases-thiocyanate-hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect Immun. 1976 Mar;13(3):800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B. Review of the progress of dairy science: antimicrobial systems in milk. J Dairy Res. 1978 Feb;45(1):131–147. doi: 10.1017/s0022029900016290. [DOI] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray C., McLaren I. A note on the effect of the lactoperoxidase systems on salmonellas in vitro and in vivo. J Appl Bacteriol. 1987 Feb;62(2):115–118. doi: 10.1111/j.1365-2672.1987.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Saito H., Tomioka H., Jidoi J. Relationship between the susceptibility of various bacteria to active oxygen species and to intracellular killing by macrophages. J Gen Microbiol. 1987 Aug;133(8):2015–2021. doi: 10.1099/00221287-133-8-2015. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Saito H., Tomioka H., Jidoi J. Susceptibility of micro-organisms to active oxygen species: sensitivity to the xanthine-oxidase-mediated antimicrobial system. J Gen Microbiol. 1987 Aug;133(8):2007–2014. doi: 10.1099/00221287-133-8-2007. [DOI] [PubMed] [Google Scholar]



