Abstract
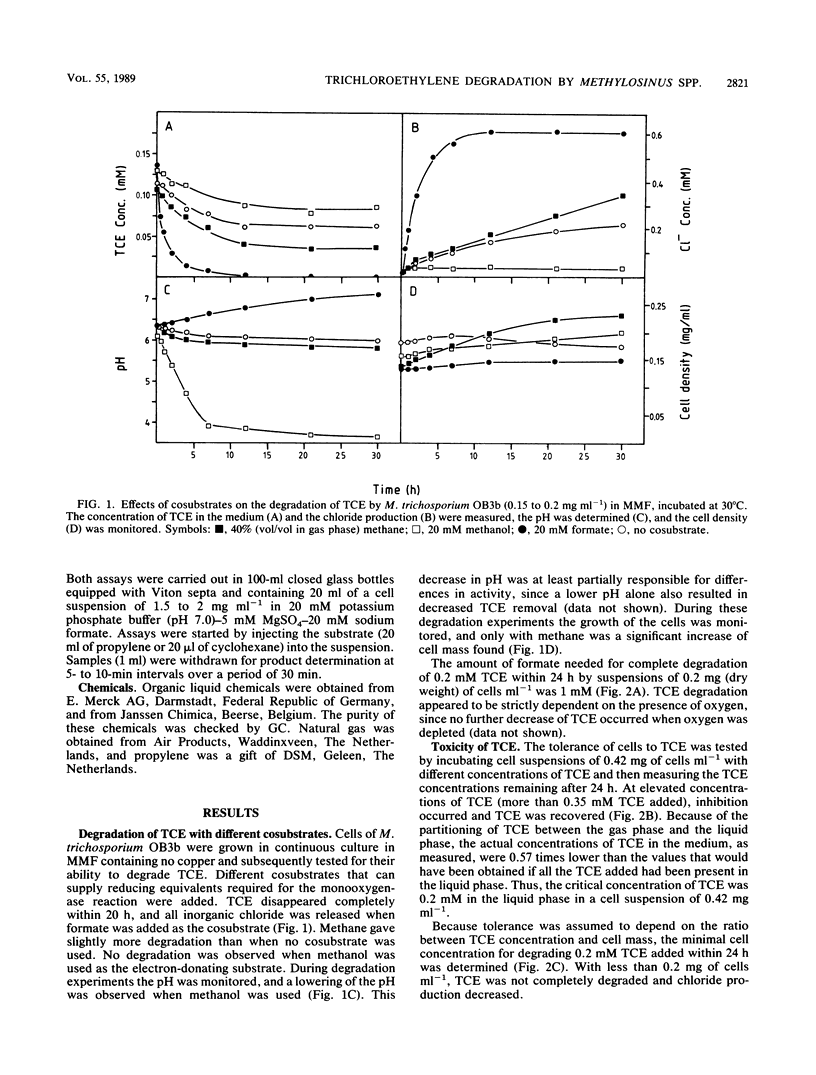
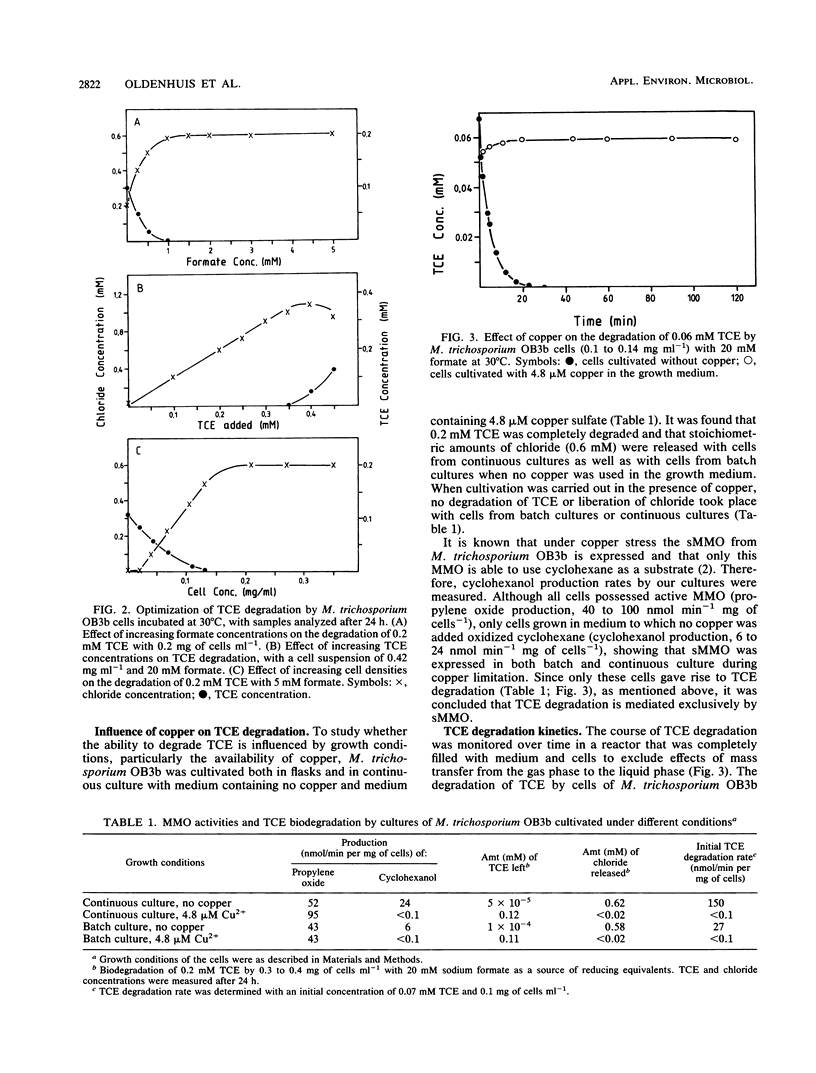
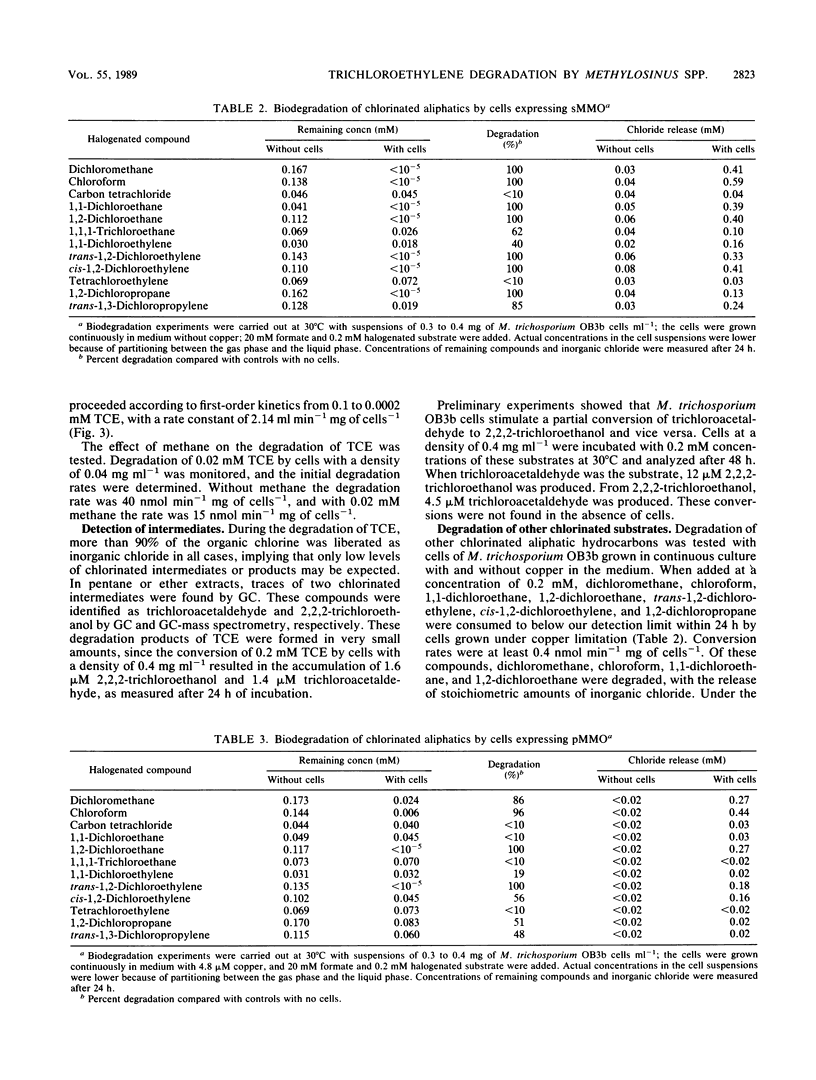
Degradation of trichloroethylene (TCE) by the methanotrophic bacterium Methylosinus trichosporium OB3b was studied by using cells grown in continuous culture. TCE degradation was a strictly cometabolic process, requiring the presence of a cosubstrate, preferably formate, and oxygen. M. trichosporium OB3b cells degraded TCE only when grown under copper limitation and when the soluble methane monooxygenase was derepressed. During TCE degradation, nearly total dechlorination occurred, as indicated by the production of inorganic chloride, and only traces of 2,2,2-trichloroethanol and trichloroacetaldehyde were produced. TCE degradation proceeded according to first-order kinetics from 0.1 to 0.0002 mM TCE with a rate constant of 2.14 ml min-1 mg of cells-1. TCE concentrations above 0.2 mM inhibited degradation in cell suspensions of 0.42 mg of cells ml-1. Other chlorinated aliphatics were also degraded by M. trichosporium OB3b. Dichloromethane, chloroform, 1,1-dichloroethane, and 1,2-dichloroethane were completely degraded, with the release of stoichiometric amounts of chloride. trans-1,2-Dichloroethylene, cis-1,2-dichloroethylene, and 1,2-dichloropropane were completely converted, but not all the chloride was released because of the formation of chlorinated intermediates, e.g., trans-2,3-dichlorooxirane, cis-2,3-dichlorooxirane, and 2,3-dichloropropanol, respectively. 1,1,1-Trichloroethane, 1,1-dichloroethylene, and 1,3-dichloropropylene were incompletely converted, and the first compound yielded 2,2,2-trichloroethanol as a chlorinated intermediate. The two perchlorinated compounds tested, carbon tetrachloride and tetrachloroethylene, were not converted.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M. M., Taddeo A. R., Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986 Apr;51(4):720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Dalton H. Steady-state kinetic analysis of soluble methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1986 May 15;236(1):155–162. doi: 10.1042/bj2360155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C. New findings in methane-utilizing bacteria highlight their importance in the biosphere and their commercial potential. Nature. 1980 Aug 7;286(5773):561–564. doi: 10.1038/286561a0. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Scheper A., Dijkhuizen L., Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985 Mar;49(3):673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Oxidation of trichloroethylene by liver microsomal cytochrome P-450: evidence for chlorine migration in a transition state not involving trichloroethylene oxide. Biochemistry. 1982 Mar 2;21(5):1090–1097. doi: 10.1021/bi00534a041. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Mahaffey W. R., Pritchard P. H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987 May;53(5):949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Colby J., Dalton H. A comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from three strains of methane-oxidizing bacteria. Biochem J. 1979 Jan 1;177(1):361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand S. E., Shippert L. Oxidation of chloroform in an aerobic soil exposed to natural gas. Appl Environ Microbiol. 1986 Jul;52(1):203–205. doi: 10.1128/aem.52.1.203-205.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson B. H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985 Jan;49(1):242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



