Abstract
(3aS,4S,6R,6aR)-Tetrahydro-2,2-dimethyl-6-vinyl-3aH-cyclopenta[d][1,3]-dioxol-4-ol, itself available from ribose, provided a convenient entry point for an 18-step preparation of carbocyclic sinefungin. This procedure is adaptable to a number of carbocyclic sinefungin analogs with diversity of heterocyclic base and in the amino acid bearing side chain.
Keywords: stereospecific allylboration, pyrazine protected aminoacid, Horeau method
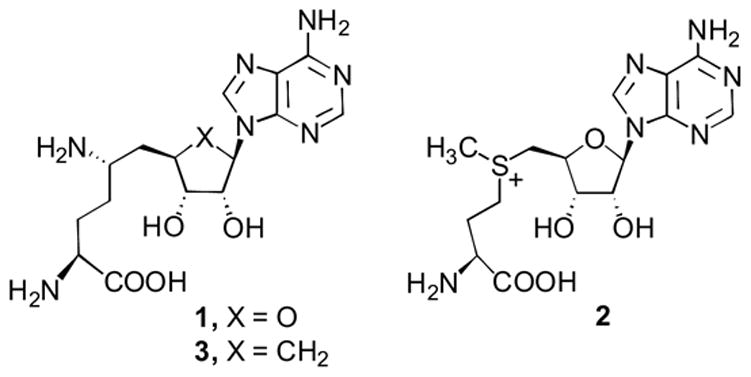
Sinefungin (1)1 is an amino acid-containing nucleoside isolated from the cultures of Streptomyces griseolus2a and Streptomyces incarnatus.2b The C-6′ primary amino center renders sinefungin structurally similar to S-adenosylmethionine (2, AdoMet). This resemblance has served as the mechanistic focal point for rationalizing sinefungin’s in vivo and in vitro biological activities, including antiviral,3–5 antifungal,2,6 amoebicidal,7 and antiparasitical,8 through inhibition of, primarily,3 AdoMet-dependent methyltransfrases.4 However, the clinical promise of 1 is restricted by its in vivo toxicity.9
In our antiviral drug discovery program sinefungin represents an important target for structural modification in order to improve its therapeutic index. Among the many compounds, which have been synthesized and evaluated in the sinefungin series,10 carbocyclic sinefungin (3) has been proven to be elusive.11 This communication discloses a practical synthesis of 3 that is adaptable to analog development.
A retrosynthetic analysis of carbocyclic sinefungin led us to a convergent approach involving a purine base and an appropriately crafted (stereochemically and functionally) cyclopentane. Thus, protection of the secondary alcohol of 412 to 5 was followed by hydroboration to provide the primary alcohol 6. Oxidation of 6 by a modified Swern procedure gave aldehyde 7. Calling on the Brown allylboration13 7 produced 8 in consistent yields (de 90% by NMR).
The side-chain stereochemistry of 8 was clarified by a modified Horeau method14 using 2-phenylbutryl chloride, pyridine and DMAP as reagents. The recovered optically active 2-phenylbutanoic acid was levorotatory. Thus,14b the homoallylic configuration of 8 is S. This result is consistent with the si face selectivity for the Brown allylboration conditions used.13
Mesylation of 8 followed by sodium azide nucleophilic substitution produced 9. Transformation of 9 into the azide-alcohol 10 was accomplished by sodium periodate glycolization/cleavage with, subsequent, Luche reduction (NaBH4/CeCl3·7 H2O).15 (It is to be noted that use of NaBH4 alone in the last step of the 9 to 10 conversion led to an intractable mixture of two products.16)
Derivative 10 was readily converted into the iodide 11 using the reagent obtained from iodine-imidazole. The lithium salt of (3R)-3,6-dihydro-2,5-dimethoxy-3-isopropylpyrazine reacted with 11 in the presence of Cu(I)17 to provide the requisite 12 as one diastereomer (by NMR). Oxidative deprotection of the PMB ether group of 12 yielded 13.
Use of the Mitsunobu reaction18 to construct the purine conjugate (that is, with 13 and 6-chloropurine) was successful but the subsequent ammonolysis at the purine C-6 center yielded mostly decomposed materials. Thus, a more traditional nucleophilic coupling process was undertaken by derivatizing 13 as its triflate that was, in turn, treated with the sodium salt of adenine to yield 14. Hydrolytic (acidic) removal of the pyrazine and isopropylidene units followed by azide reduction and saponification (of the methyl ester made available by breakdown of the pyrazine ring) led to achievement of carbocyclic sinefungin (3).19
Figure.
Scheme.
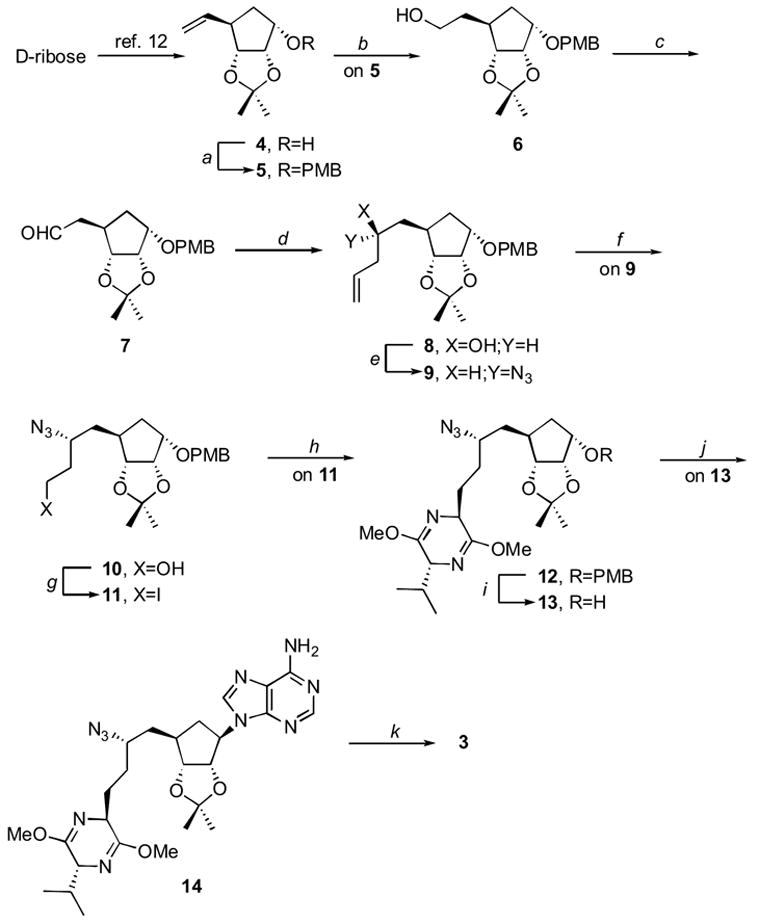
Reagents and conditions: a, PMBCl, NaH, DMF, 95%; b, (i) 9-BBN, THF; (ii) MeOH, H2O2, NaOH, 98% for two steps; c, SO3·py, DMSO, DIPEA, CH2Cl2, 94%; d, (i) (+)-B-methoxydiisopinocampheylborane, CH2=CHCH2MgBr, Et2O/THF; (ii) MeOH, H2O2, NaOH, 96% for two steps; e, (i) MsCl, Et3N, DMAP, CH2Cl2; (ii) NaN3, DMF, 85% for two steps; f, (i) NaIO4, OsO4, MeOH/H2O; (ii) NaBH4, CeCl3·7 H2O, MeOH, 77% for two steps; g, TPP, imidazole, I2, toluene/MeCN, 90%; h, (3R)-3,6-dihydro-2,5-dimethoxy-3-isopropylpyrazine, BuLi, CuCN, THF, 87%; i, DDQ, CH2Cl2/H2O, 88%; j, (i) Tf2O, pyridine, CH2Cl2; (ii) adenine, NaH, DMF, 45% for two steps; k, (i) 0.5 N HCl MeOH; (ii) Pd(OH)2/C, cyclohexene; (iii) LiOH, MeOH/H2O, 55% for three seps.
Acknowledgments
This research was supported by funds from the NIH (AI 56540). The preliminary investigations of Tetyana Shulyak and Minmin Yang of the Auburn group assisted in the design of the successful synthesis described here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghosh AK, Liu W. J Org Chem. 1996;61:6175. doi: 10.1021/jo960670g. and references cited therein. Mucha A, Cappanelli M, Szczepanik W, Kaczmarek P, Skaa J, Jezowska-Bojczuk M. J Inorg Biochem. 2006;100:178. doi: 10.1016/j.jinorgbio.2005.11.001.
- 2.(a) Hamill RL, Hoehn MM. J Antibiot. 1973;26:463. doi: 10.7164/antibiotics.26.463. [DOI] [PubMed] [Google Scholar]; (b) Florent J, Lunel J, Mancy D. 4,189,349. US Patent. 1980
- 3.Nagarajan R. 4,158,056. US Patent. 1979
- 4.(a) Pugh CS, Borchardt RT. Biochemistry. 1982;21:1535. doi: 10.1021/bi00536a011. [DOI] [PubMed] [Google Scholar]; (b) Pugh CSG, Borchardt RT, Stone HO. J Biol Chem. 1978;253:4075. [PubMed] [Google Scholar]
- 5.Vedel M, Lawrence F, Robert-Gero M, Lederer E. Biochem Biophys Res Commun. 1978;85:371. doi: 10.1016/s0006-291x(78)80052-7. [DOI] [PubMed] [Google Scholar]
- 6.Hamill RL, Nagarajan R. 4,087,603. US Patent. :1976.
- 7.Ferrante A, Ljungström L, Huldt G, Lederer E. Trans Roy Soc Trop Med Hyg. 1984;78:837. doi: 10.1016/0035-9203(84)90039-7. [DOI] [PubMed] [Google Scholar]
- 8.Trager W, Tershacovec M, Chiang PK, Cantoni GL. Exp Parasitol. 1980;50:83. doi: 10.1016/0014-4894(80)90010-7. [DOI] [PubMed] [Google Scholar]
- 9.Zwyegarth E, Schillinger D, Kaufmann W, Röttcher D. Trop Med Parasitol. 1986;37:255. [PubMed] [Google Scholar]
- 10.See for example, Barton DHR, Gero SD, Lawrence F, Robert-Gero M, Quiclet-Sire B, Samadi M. J Med Chem. 1992;35:63. doi: 10.1021/jm00079a007.Barton DHR, Gero SD, Negron G, Quiclet-Sire B, Samadi M, Vincent C. Nucloesides Nucleotides. 1995;14:1619.Blanchard P, Dodic N, Fourrey JL, Lawrence F, Mouna AM, Robert-Gero M. J Med Chem. 1991;34:2798. doi: 10.1021/jm00113a018.Marasco CJ, Jr, Kramer DL, Miller J, Porter CW, Bacchi CJ, Rattendi D, Kucera L, Iyer N, Bernacki R, Pera P, Sufrin JR. J Med Chem. 2002;45:5112. doi: 10.1021/jm0201621.Maria EJ, da Silva AD, Fourrey JL. Eur J Org Chem. 2000:627–631.Secrist JA, III, Talekar RR. Nucleosides Nucleotides. 1990;9:619.Lyga JW, Secrist JA., III J Org Chem. 1983;48:1982.Peterli-Roth P, Maguire MP, Leon E, Rapoport H. J Org Chem. 1994;59:4186.
- 11.(a) Gage JL, Priour AA, Savela GC, Miller MJ. Abstracts of Papers, 216th National Meeting of the American Chemical Society; Boston, MA. August 23–27, 1998; Washington, DC: American Chemical Society; 1998. ORGN 314. [Google Scholar]; (b) Da Silva AD, Maria EJ, Blanchard P, Fourrey JL, Robert-Gero M. Nucleosides Nucleotides. 1998;17:2175. [Google Scholar]; (c) Miller MJ, Jin B, Warshakoon NC, Gage JL. Abstracts of Papers, 226th National Meeting of the American Chemical Society; New York, NY. September 7–11, 2003; Washington, DC; ORGN 589: American Chemical Society; [Google Scholar]; (d) Jiang MXW, Jin B, Gage JL, Priour A, Savela G, Miller MJ. J Org Chem. 2006;71:4164. doi: 10.1021/jo060224l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Ye W, Schneller SW. J Org Chem. 2004;69:3993. doi: 10.1021/jo040119g. [DOI] [PubMed] [Google Scholar]
- 13.(a) Brown HC, Jadhav PK. J Am Chem Soc. 1983;105:2092. [Google Scholar]; (b) Brown HC, Bhat KS. J Am Chem Soc. 1986;108:5919. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 14.(a) Horeau A, Kagan HB. Tetrahedron. 1964;20:2431. doi: 10.1016/s0040-4020(01)90823-3. [DOI] [PubMed] [Google Scholar]; (b) Barnekow DE, Cardellina JH., II Tetrahedron Lett. 1989;30:3629. [Google Scholar]
- 15.Luche JL. J Am Chem Soc. 1978;100:2226. [Google Scholar]
- 16.Boyer JH, Ellzey SE., Jr J Org Chem. 1958;23:127. [Google Scholar]
- 17.Baldwin JE, Adlington RM, Mitchell MB. Tetrahedron. 1995;51:5193. [Google Scholar]
- 18.(a) Mitsunobu O. Synthesis. 1981:1. [Google Scholar]; (b) Hughes DL. Org Prep Proced Int. 1996;28:127. [Google Scholar]
- 19.Selected data for 3: white foam; 1H NMR (D2O, 250 MHz) δ 8.23 (s, 1H), 8.18 (s, 1H), 4.80 (d, J=2.8 Hz, 1H), 4.58 (m, 1H), 4.03 (m, 2H), 3.70 (m, 1H), 2.56 (m, 1H), 2.26-1.73 (m, 8H); 13C NMR (D2O, 100 MHz) δ 188.5, 158.1, 154.9, 151.8, 143.4, 121.4, 77.42, 77.38, 62.54, 62.47, 52.9, 42.4, 42.3, 34.9, 27.06, 27.00; HRMS calcd for C16H24N6O5 [M-H2O+H+] 362.1945. Found: 362.1941.


