Abstract
The regioselective synthesis of 3,5-disubstituted isoxazoles was achieved through the 1,3-dipolar cycloaddition of nitrile oxides with 1,1-disubstituted bromoalkenes. The substituted bromoalkenes function as alkyne synthons which were used to construct 5,5-disubstituted bromoisoxazoline intermediates that aromatize to the analogous isoxazoles through the loss of HBr.
Keywords: Cycloaddition, Regioselectivity, Heterocycles, Alkyne Surrogate, Dehydrohalogenation
Since a number of isoxazoles display anti-inflammatory1, antiviral,2 as well as antitubulin3 activity, the synthesis of this family of heterocycles continues to be of interest. One of the most frequently used methods to synthesize isoxazoles is a 1,3-dipolar cycloaddition involving a nitrile oxide, and the usual dipolarophile for this process is an alkyne.4 Although some alkynes are commercially available, the synthesis of many functionalized alkynes can take two or more steps. Additionally, the 1,3-dipolar cycloaddition of alkynes, with a few exceptions,5 often lead to a mixture of regioisomeric products.4,6b The use of alkyne synthons6 can serve to alleviate many of the alkyne preparatory and cycloaddition regioselectivity issues.7 These alkyne surrogates are usually alkenes that have a functional group that can be eliminated in situ during cycloaddition.6 Herein we report the application of 1,1-disubstituted bromoalkenes as alkyne equivalents for the regioselective synthesis of 3,5-disubstituted isoxazoles via 1,3-dipolar cycloaddition.
Scheme 1.
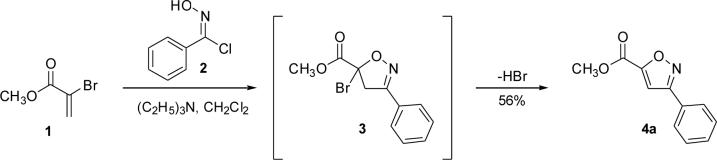
Isoxazole synthesis from 2-bromo-acrylic acid methyl ester through a 5-bromoisoxazoline intermediate.
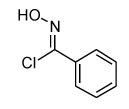
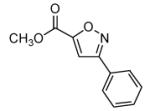
During our study of the synthesis of functionalized 5,5-disubstituted isoxazolines, we discovered that when 2-bromo-acrylic acid methyl ester (1) was used as the alkene, isoxazole (4) was isolated as the sole product instead of the bromoisoxazoline (3, Scheme 1).8 The most probable driving force for the formation of 4 is the creation of a stable aromatic system through the loss of HBr. Alternatively, since the reaction conditions are basic, it is quite possible for the bromoalkene, 1, to decompose to the corresponding alkyne before reacting with the nitrile oxide. In order to rule out this reaction pathway, we exposed 1 to triethylamine for 24 hours, and no decomposition or formation of alkyne was observed. The experimental data point to the formation of 3 followed by its aromatization to 4,6 and the regioselectivity is in accordance with both steric and frontier molecular orbital interactions of the 1,3-dipole and the alkene.9 The study of the 1,3-dipolar cycloaddition reaction of compound 1 and other bromoalkenes was undertaken in order to determine the general efficacy of these bromoalkenes as alkyne substitutes. The results of the cycloaddition of 1 and 3-bromo-but-3-en-2-one with three different nitrile oxides are shown in Table 1. All of these cycloadditions occurred with complete regiochemical integrity10 in reasonable to good isolated yields.
Table 1.
Isoxazoles formed from the 1,3-dipolar cycloaddition reactions using carbonyl containing bromoalkenes as the dipolarophile.
| Entry | Alkene | α-Chloro oxime | Product | % Yield |
|---|---|---|---|---|
| 1 | 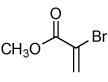 |
 |
 |
79 |
| 2 | 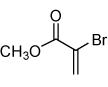 |
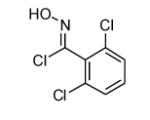 |
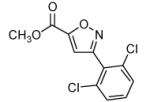 |
68 |
| 3 | 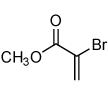 |
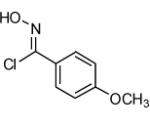 |
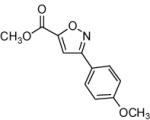 |
64 |
| 4 | 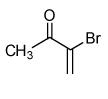 |
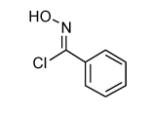 |
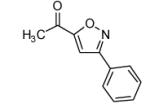 |
75 |
| 5 | 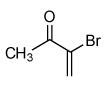 |
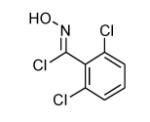 |
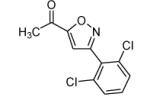 |
80 |
| 6 | 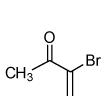 |
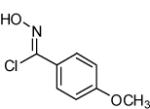 |
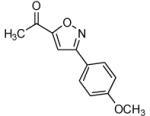 |
80 |
Interestingly, the major isolated cycloadduct from the reaction of nitrile oxides with phenyl sulfone containing alkynes is the 4-substituted isoxazole4 which is contrary to the observed regioselectivity with carbonyl alkynes.6b To test this influence on the regioselectivity of the sulfone, a phenyl sulfone containing bromoalkene was subjected to the 1,3-dipolar cycloaddition reaction with three different 1,3-dipoles. As shown in table 2, the exclusive isolated regioisomer is the isoxazole with the phenyl sulfone in the 5-position. Under certain reaction conditions, phenyl sulfoxides can serve as leaving groups.11 Therefore, a phenyl sulfoxide bromoalkene was reacted with three different nitrile oxides in order to determine if the phenyl sulfoxide would compete with the bromide ion as a leaving group. The cycloaddition occurred with complete regioselectivity, and the 5-benzenesulfinyl isoxazole was isolated with no evidence of the 5-bromoisoxazole as shown in table 2.10,12
Table 2.
Isoxazoles formed from the 1,3-dipolar cycloaddition reactions using sulfone and sulfoxide containing bromoalkenes as the dipolarophile.
| Entry | Alkene | α-Chloro oxime | Product | % Yield |
|---|---|---|---|---|
| 7 | 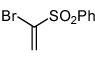 |
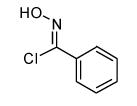 |
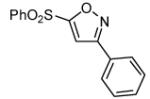 |
91 |
| 8 | 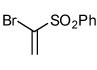 |
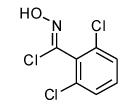 |
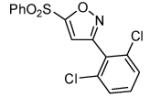 |
85 |
| 9 | 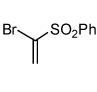 |
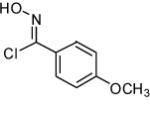 |
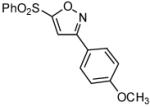 |
81 |
| 10 | 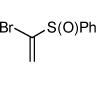 |
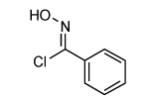 |
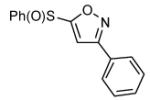 |
91 |
| 11 | 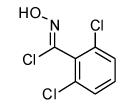 |
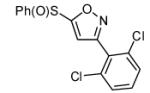 |
69 | |
| 12 | 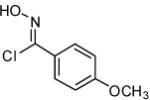 |
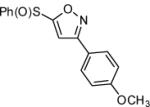 |
71 |
In summary, this study shows the application of 1,1-disubstituted bromoalkenes as alkyne synthons that allow for direct and regioselective synthesis of isoxazoles through 1,3-dipolar cycloaddition. Future investigations designed to extend this methodology towards isoxazole synthesis from a mono substituted alkene and an α-chlorobenzaldoxime in one reaction vessel are currently underway.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (G12RR13459) and the National Science Foundation (HRD-0401730) for generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data.
The supplementary data is available online with the paper in ScienceDirect.
References and Notes
- 1.Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J. Med. Chem. 2000;43:775. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lee Y-S, Kim BH. Bioorg. Med. Chem. Lett. 2002;12:1395. doi: 10.1016/s0960-894x(02)00182-8. [DOI] [PubMed] [Google Scholar]; (b) Srivastava S, Bajpai LK, Batra S, Bhaduri AP, Maikhuri JP, Gupta G, Dhar JD. Bioorg. Med. Chem. 1999;7:2607. doi: 10.1016/s0968-0896(99)00188-1. [DOI] [PubMed] [Google Scholar]
- 3.(a) Simoni D, Grisolia G, Giannini G, Roberti M, Rondanin R, Piccagli L, Baruchello R, Rossi M, Romagnoli R, Invidiata FP, Grimaudo S, Jung MK, Hamel E, Gebbia N, Crosta L, Abbadessa V, Di Cristina A, Dusonchet L, Meli M, Tolomeo M. J. Med. Chem. 2005;48:723. doi: 10.1021/jm049622b. [DOI] [PubMed] [Google Scholar]; (b) Kaffy J, Pontikis R, Carrez D, Croisy A, Monneret C, Florent J-C. Bioorg. Med. Chem. 2006;14:4067. doi: 10.1016/j.bmc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sandanayaka VP, Youjun Y. Org. Lett. 2000;3087;2 doi: 10.1021/ol006256r. [DOI] [PubMed] [Google Scholar]; (b) Croce PD, La Rosa C, Zecchi G. J. Chem. Soc. Perkin Trans. 1985;1:2621. [Google Scholar]
- 5.Stang PJ, Murch P. Tetrahedron Lett. 1997;38:8793. For examples of highly regioselective 1,3-dipolar cycloadditions with of alkynliodonium salts please see: [Google Scholar]
- 6.(a) Easton CJ, Hughes CM, Tiekink ERT, Lubin CE, Savage GP, Simpson GW. Tetrahedron Lett. 1994;35:3589. [Google Scholar]; (b) Easton CJ, Heath GA, Hughes CMM, Lee CKY, Savage GP, Simpson GW, Tiekink ERT, Vuckovic GJ, Webster RD. J. Chem. Soc. Perkin Trans. 2001;1:1168. [Google Scholar]; (c) Jimenez R, Perez L, Tamariz J, Salgado H. Heterocycles. 1993;35:591. [Google Scholar]
- 7. The reported regioselectivites for 1,3-dipolar cycloaddition of similar terminal carbonyl alkynes range from 3:1 to 6:1 favoring the 5-isoxazole, and 4-isoxazoles are preferred over the 5-isoxazole (2:1 to 4:1) for comparable terminal phenyl sulfone alkynes.
- 8.Hamme AT, II, Xu J, Wang J, Cook T, Ellis E. Heterocycles. 2005;65:2885. [Google Scholar]
- 9.(a) Houk KN, Sims J, Duke RE, Jr., Strozier RW, George JK. J. Am. Chem. Soc. 1973;95:7287. [Google Scholar]; (b) Houk KN, Sims J, Watts CR, Luskus LJ. J. Am. Chem. Soc. 1973;95:7301. [Google Scholar]; (c) Houk KN. Acc. Chem. Res. 1975;8:361. [Google Scholar]; (d) Padwa A. 1,3-Dipolar Cycloaddition Chemistry. John Wiley & Sons; New York: 1984. [Google Scholar]
- 10. All of the isolated products for this publication were unambiguously characterized by 1H and 13C NMR, IR, and HRMS, and for regiochemical assignment, all analytical data is consistent with literature precedent.
- 11.(a) Miyaoka H, Kajiwara Y, Hara Y, Yamada Y. J. Org. Chem. 2001;66:1429. doi: 10.1021/jo0015772. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Bridges AJ. J. Org. Chem. 1975;40:2014. [Google Scholar]
- 12. General Procedure: A solution of the bromoalkene (0.25 mmol) and the hydroximinoyl chloride (0.25 mmol) in 5 mL dry dichloromethane was treated with triethylamine (28 mg, 0.275 mmol). The reaction mixture was stirred at rt until the disappearance of the starting materials, as evidenced by TLC. After the reaction was complete, a minimum amount of silica gel was added, and the solvent was evaporated under reduced pressure. The crude products were purified by flash column chromatography over silica gel using hexanes-ethyl acetate (4:1) as an eluant system.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.