Summary
Neuregulin1 (NRG1) has been strongly linked genetically to schizophrenia although the pathophysiological role it plays in the disease is not known. The prevailing models of schizophrenia invoke hypofunction of the glutamatergic synapse as well as defects in early development of the hippocampal – cortical circuitry. Here we show that erbB4, as a postsynaptic target of NRG1, plays a key role in activity-dependent maturation and plasticity of excitatory synaptic structure and function. Synaptic activity leads to the activation and recruitment of erbB4 into the synapse. Overexpressed erbB4 selectively enhances AMPA synaptic currents and increases dendritic spine size. Preventing NRG1/ErbB4 signaling destabilizes synaptic AMPA receptors and leads to loss of synaptic NMDA currents and spines. Our results indicate that normal activity-driven glutamatergic synapse development is impaired by genetic deficits in NRG1/erbB4 signaling leading to glutamatergic hypofunction. These findings link NRG1/erB4 function to the major models proposed for the etiology of schizophrenia.
Introduction
The function of NRG1 in the brain has gained much attention since the initial discovery (Stefansson et al., 2002) and subsequent confirmation (Harrison and Law, 2006; Harrison and Weinberger, 2005; Scolnick et al., 2006) that nrg1 gene is linked to schizophrenia..
Further studies showed that erbB4, a receptor of NRG1, is also associated with schizophrenia (Law et al., 2007; Norton et al., 2006a; Silberberg et al., 2006). A change in the expression level of NRG1 isoforms (Hashimoto et al., 2004; Law et al., 2006; Petryshen et al., 2005) or erbBs (Hakak et al., 2001; Silberberg et al., 2006; Sugai et al., 2004) has been reported in schizophrenia patients. These studies strongly support the hypothesis that a perturbation in NRG1 signaling in the brain can contribute to the etiology of schizophrenia.
NRG1 has multiple biological functions (Falls, 2003). In the peripheral nervous system (PNS), it regulates target cell differentiation (Hippenmeyer et al., 2002), neurotransmitter receptor expression (Buonanno and Fischbach, 2001; Chu et al., 1995; Falls et al., 1993; Fischbach and Rosen, 1997; Jo et al., 1995; Schaeffer et al., 2001; Usdin and Fischbach, 1986; Wolpowitz et al., 2000), NMJ or interneuronal PNS synapse development (Lin et al., 2000; Morris et al., 1999; Woldeyesus et al., 1999; Wolpowitz et al., 2000; Yang et al., 1998), and Schwann cell survival (Escher et al., 2005; Kummer et al., 2006).
In the brain, NRG1 signaling regulates radial glia formation and neuronal migration (Anton et al., 1997; Rio et al., 1997), oligodendrocyte development and axon myelination (Calaora et al., 2001; Canoll et al., 1996; Fernandez et al., 2000; Schmucker et al., 2003; Vartanian et al., 1994; Vartanian et al., 1999), axon path finding (Lopez-Bendito et al., 2006), dendritic development (Gerecke et al., 2004; Rieff and Corfas, 2006), and the expression of neurotransmitter receptors (Liu et al., 2001; Okada and Corfas, 2004; Ozaki et al., 1997; Rieff et al., 1999; Stefansson et al., 2002; Xie et al., 2004).
NRG1 function is largely mediated by a class of receptor tyrosine kinases including erbB2, erbB3, and erbB4 (Falls, 2003; Yarden and Sliwkowski, 2001). ErbB4 is likely to be the major mediator of NRG1 functions in the brain, especially for those related to schizophrenia, since first, erbB4, but not erbB2 or erbB3 mutant animals share many neural and behavioral defects with NRG1 mutants (Falls, 2003; Stefansson et al., 2002); second, erbB4 gene, but not erbB2 or 3, has been shown to associate with schizophrenia (Law et al., 2007; Norton et al., 2006a; Silberberg et al., 2006); third, altered NRG1/erbB4 signaling has been reported in the brain of schizophrenia patients (Hahn et al., 2006).
In many brain areas, NRG1 is expressed at synaptic regions (Chaudhury et al., 2003; Law et al., 2004; Ozaki et al., 2000), and its processing can be regulated by neuronal activity (Bao et al., 2003; Eilam et al., 1998; Ozaki et al., 2004). Recent biochemical studies showed that erbB4 is highly enriched in the postsynaptic density (PSD) of excitatory synapses and interacts with PSD-95 (Garcia et al., 2000; Huang et al., 2000), a major scaffolding protein in the excitatory synapse that can control synaptic function (Ehrlich and Malinow, 2004; El-Husseini et al., 2000), suggesting an important role of erbB4 in these synapses. Moreover, In the hippocampus, NRG1 mRNA is highly expressed in the CA3 area, a region presynaptic to the CA1 area which displays erbB4 expression (Law et al., 2004; Okada and Corfas, 2004). These findings suggest that NRG1/erbB4 signaling might be important for the function or development of glutamatergic synapse and circuitry in the brain.
Here, by manipulating NRG1/erbB4 signaling at the hippocampal CA3 – CA1 pathway, we demonstrate that postsynaptic erbB4 controls activity-dependent maturation and plasticity of excitatory synaptic structure and function. In particular, NRG/erbB4 signaling is positively regulated by synaptic activity. In turn, NRG/erbB4 signaling is also required for activity-dependent AMPA receptor synaptic incorporation and stabilization as well as maintenance of spine structure. As a result, interruption of signaling from presynaptic NRG1 to postsynaptic erbB4 leads to impaired synaptic development and depressed glutamatergic function. Our study indicates that NRG1/erbB4 signaling plays an important role in glutamatergic synapses, and suggests a mechanism by which genetic or activity-dependent perturbation of this pathway can contribute to the etiology of schizophrenia.
Results
Neuronal activity regulates erbB4 activation and localization
Previous biochemichal studies have shown that erbB4 is enriched in the postsynaptic density (PSD) fraction of excitatory synapse in the brain (Garcia et al., 2000; Huang et al., 2000). However, it is not clear how erbB4 synaptic localization is achieved and how its localization and function are regulated. Since NRG1 processing or release is regulated by neuronal activity (Bao et al., 2003; Eilam et al., 1998; Han and Fischbach, 1999; Ozaki et al., 2004), we hypothesized that neuronal activity also regulates erbB4 activation and its recruitment and stabilization in the synapse. We predicted that synaptic erbB4 should have a higher phosphorylation level than extrasynaptic erbB4, and changing neuronal activity should change erbB4 phosphorylation and distribution. As expected, erbB4 isolated from the PSD fraction displayed a greater degree of phosphorylation than erbB4 in the brain homogenate (homogenate: 1±0.05; PSD: 4.1±0.55; n=4 experiments; p<0.01; Fig. 1A), indicating synaptic erbB4 is preferentially activated. Moreover, increasing neuronal activity by treating hippocampal slices with picrotoxin for 1 hour markedly increased erbB4 phosphorylation in the PSD (control: 1±0.1; picrotoxin: 5±0.3; n=4 experiments; p<0.01; Fig. 1B), whereas inhibiting neuronal activity by elevated concentration of MgCl2 (Zhu et al., 2000) or TTX for 1 hour significantly decreased erbB4 phosphorylation in the PSD (control: 1±0.12; MgCl2: 0.23±0.08; TTX: 0.16±0.06; n=3 experiments; p<0.01 for both MgCl2 and TTX compared with control; Fig. 1C, D). Interestingly, inhibition of neuronal activity also reduced the total amount of erbB4 in the PSD fraction (Fig. 1C, E).
Figure 1. Activity-dependent regulation of ErbB4 phosphorylation and localization in the synapse.
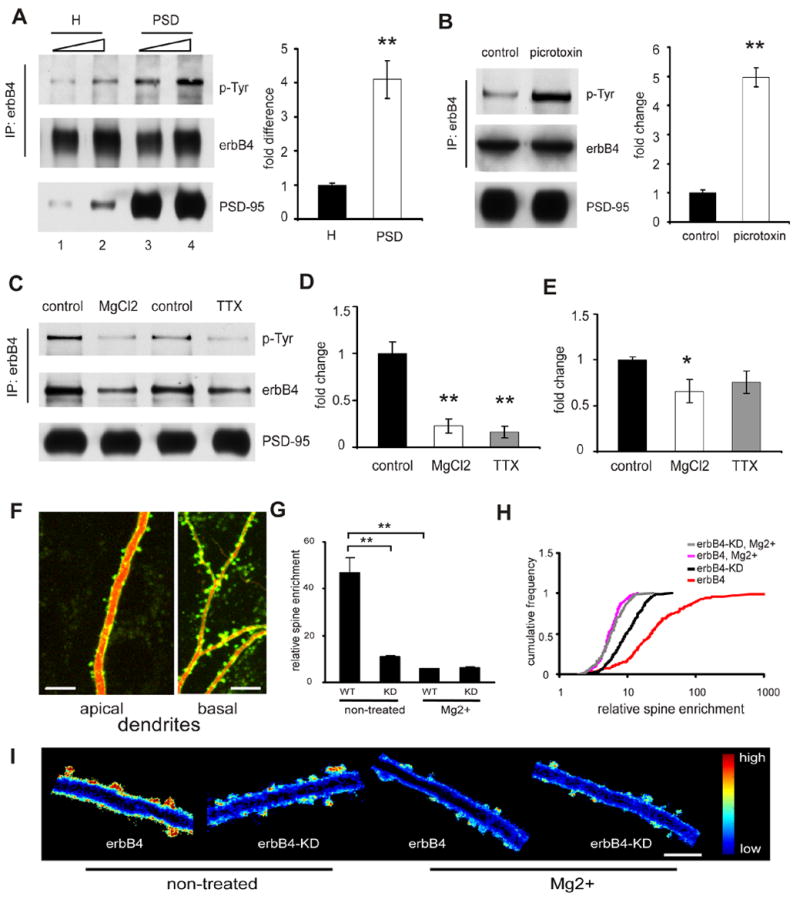
A. Enrichment of phosphorylated ErbB4 in PSD. ErbB4 was precipitated with anti-erbB4 antibodies from either brain homogenates (H) or PSD fraction. Samples were adjusted to reveal equal amounts of precipitated ErbB4 (middle panel; lanes 1 versus 3, and 2 versus 4). Precipitated ErbB4 was resolved on SDS-PAGE and immunoblotted with anti-phosphotyrosine antibodies (upper panel). Equal amounts of protein were also loaded from either homogenates or PSD fraction (lower panel; lanes 1 versus 3, and 2 versus 4) for detection of PSD-95 as a control for successful isolate of PSD fraction. Phosphoralyted erbB4 in PSD was quantified and normalized as fold difference compared to that in H (bar graph on the right side). B, C and D. Phosphorylation of ErbB4 in PSD is activity dependent. ErbB4 was precipitated from PSD isolated from brain slices and examined by western blot as in A. B: Picrotoxin (100 uM) treated sample showed elevated erbB4 phosphorylation. Quantification is on the right side. C and D: Treatment with either TTX or elevated Mg2+ resulted in a reduction of erbB4 phosphorylation in PSD, and also a reduction of total amount of erbB4 (E) in PSD. F. Representative images of the dendritic branches of a hippocampal CA1 pyramidal neuron transfected with SEP-erbB4 (green) together with tomato dsRed (red) acquired with TPLSM. ErbB4 is highly enriched in spines. Scale bar: 10 μm. G. Quantification of enrichment of SEP-erbB4 and SEP-erbB4-KD in spines after different treatment (**P<0.01 by K-S test). H. Same data as in G displayed as cumulative distribution. I. Ratio images (green/red) of cells expressing tomato dsRed and indicated SEP-tagged receptor are shown for different conditions. Blue depicts low receptor density, and red depicts high density. Scale bar: 5 μm.
To directly visualize the subcellular distribution of erbB4, we tagged recombinant erbB4 in the extracellular region with a pH sensitive form of GFP, super-ecliptic pHluorin (SEP), which we have previously shown to be a marker for receptors on the cell surface (Kopec et al., 2006). After transfection of CA1 pyramidal cells in organotypic hippocampal slices with SEP-erbB4 together with a red fluorescent protein, tomato dsRed, as a cellular marker, the subcellular distribution of erbB4 was examined by dual channel two-photon laser-scanning microscopy (TPLSM) (Kopec et al., 2006). We found that SEP-erbB4 is highly enriched on the surface of spines of CA1 pyramidal cells (Fig. 1F, G, and H).
Consistent with the biochemical results, our imaging data also showed that erbB4 subcellular localization is regulated by neuronal activity. When transfected slices are maintained in normal media, SEP-erbB4 is highly enriched on the surface of spines. However, maintaining slices with elevated concentration of Mg2+ markedly reduced this enrichment (normal media: 47±6.3, n=212 spines from 3 cells; Mg2+ treated: 5.9±0.1, n=266 spines from 4 cells; P<0.01; Fig. 1G, H, and I). To test if erbB4 activation is required for its spine enrichment, we expressed an erbB4 mutant that lacks the kinase activity due to a point mutation in the kinase domain, which has been shown to function as a dominant negative erbB4 (Yang et al., 2005). The kinase-dead erbB4 (SEP-erbB4-KD) is much less enriched on the surface of spine (11.1±0.3, n=417 spines from 4 cells; P<0.01 compared with SEP-erbB4). Treatment with Mg2+ further reduced the enrichment of SEP-erbB4-KD on the spine surface (6.4±0.2, n=163 spines from 4 cells; P<0.01 compared with non-treated SEP-erbB4-KD; Fig. 1G, H, and I).
These data indicate that neuronal activity regulates erbB4 activation, erbB4 activation is required for its synaptic recruitment or stabilization, and suggest that erbB4 plays an important role in glutamatergic synapses.
Activity-dependent erbB4 regulation of synaptic transmission
To test if erbB4 is important for synaptic function at hippocampal CA3 - CA1 synapse, we first examined the effects of erbB4 overexpression on synaptic transmission by simultaneous recordings of evoked EPSCs from a CA1 pyramidal neuron overexpressing erbB4 and an adjacent control neuron. Overexpression of either non-tagged erbB4 (together with GFP as a cellular marker) or SEP-erbB4 results in a potentiation of AMPA receptor (AMPAR) mediated transmission (control: 1±0.1; erbB4: 1.8±0.2; n=31 pairs; P<0.01), but not NMDA receptor (NMDAR) (control: 1±0.2; erbB4: 0.83±0.1; n=27 pairs; P>0.05) mediated transmission (Fig. 2A). Overexpression of erbB4 isoform CYT-1, or isoform CYT-2, which lacks a PI3-kinase binding domain, had similar effects (data not shown), suggesting that the PI3-kinase binding domain is not required for potentiating AMPAR mediated synaptic transmission.
Figure 2. erbB4 controls synaptic transmission in an activity dependent manner.
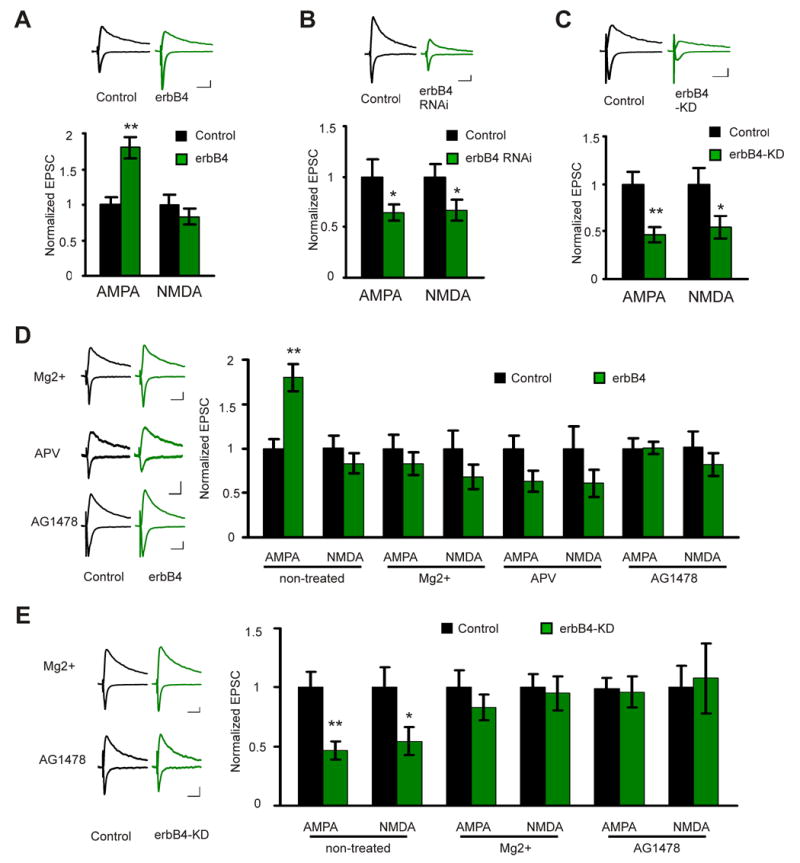
A. upper: representative traces of EPSCs recorded simultaneously from a pair of neurons (black: control neuron; green: neuron overexpressing erbB4; EPSCs from both −60 and +40 mV holding potentials are shown). Scale bars: 50 ms and 20 pA. Lower: quantification of the EPSCs mediated by both AMPAR and NMDAR from pairs of control and erbB4 overexpressing neurons. The EPSC amplitude was normalized to the mean value of EPSCs from control neurons. The same way of data display applies for all the subsequent electrophysiology data. B. Same as A, except that the recordings were made from pairs of control and erbB4 RNAi expressing neurons. C. Same as B, except that the recordings were made from pairs of control and erbB4-KD expressing neurons. D. Left: representative traces for recordings from paired control and erbB4 overexpressing neurons under MgCl2, APV, or AG1478 treated condition. Right: quantification of the EPSCs mediated by both AMPAR and NMDAR under different conditions. E. Left: representative traces for recordings from paired control and erbB4-KD expressing neurons under MgCl2 or AG1478 treated condition. Right: quantification of the EPSCs mediated by both AMPAR and NMDAR under different conditions. *P<0.05; **P<0.01. Scale bars: 50 ms and 20 pA.
To examine the function of endogenous erbB4, we employed RNA interference (RNAi) to knock down erbB4 protein level. Neurons were infected with a Lenti virus expressing, via a dual promoter system, GFP (as a marker for infected cells) and short hairpin RNAs specifically targeting different regions of erbB4 transcript. Three different short hairpin RNAs reduced erbB4 protein expression to different degrees, with hairpin number two (hp2) being most effective (supplemental Fig. 1A). After infecting CA1 cells, hp2 significantly depressed both AMPAR (control: 1±0.2; hp2: 0.65±0.1; n=10 pairs, P<0.05) and NMDAR (control: 1±0.1; hp2: 0.67±0.1, n=8 pairs, P<0.05) mediated transmission (Fig. 2B). Hp3 also depressed synaptic transmission, whereas hp1, which was less effective in knocking down erbB4 expression, failed to suppress transmission (supplemental Fig. 1B). To further test the specificity of erbB4 RNAi, hp2 was expressed together with an erbB4 construct that has silent mutations that avoid targeting by hp2 (erbB4-rescue). Like expressing erbB4 alone, expressing of hp2 together with erbB4-rescue resulted in a potentiation of AMPAR mediated transmission but no effect on NMDAR mediated transmission (supplemental Fig. 1C), indicating that erbB4 RNAi is specific.
To determine whether the kinase activity of erbB4, or other functional domains are responsible for the effects of erbB4, we tested the effects of erbB4-KD. ErbB4-KD depressed both AMPAR (control: 1±0.1; erbB4-KD: 0.47±0.1; n=16 pairs; P<0.01) and NMDAR (control: 1±0.2; erbB4-KD: 0.55±0.1; n=14 pairs; P<0.05) mediated transmission (Fig. 2C), similar to the effects of erbB4 RNAi, suggesting erbB4-KD functions as a dominant-negative. These data indicate that erbB4 controls excitatory synaptic transmission at the CA3 - CA1 synapse.
Since erbB4 activation and synaptic localization are activity dependent, it is likely that the function of NRG1/erbB4 in the glutamatergic synapse also depends on neuronal activity. To test this, we incubated organotypic hippocampal slices in medium containing elevated concentration of Mg2+, and then tested the effects of erbB4 and erbB4 KD on synaptic transmission in CA3 – CA1 synapse. Mg2+ treatment prevented the potentiation effect of erbB4 on AMPAR mediated transmission (AMPAR: control 1±0.2; erbB4 0.83±0.1, n=10 pairs, P>0.05; NMDAR: control 1±0.2; erbB4 0.68±0.1, n=9 pairs, P>0.05; Fig. 2D), and occluded the depression effects of erbB4 KD on both AMPAR and NMDAR mediated transmission (AMPAR: control 1±0.1; erbB4 0.83±0.1, n=9 pairs, P>0.05; NMDAR: control 1±0.1; erbB4 0.95±0.1, n=9 pairs, P>0.05; Fig. 2E). APV also blocked the potentiating effect of erbB4 (AMPAR: control 1±0.1; erbB4 0.63±0.1, n=8 pairs, P>0.05; NMDAR: control 1±0.2; erbB4 0.61±0.2, n=7 pairs, P>0.05; Fig. 2D), indicating that NMDAR activation is involved. Treatment with either Mg2+ or APV had a tendency to depress transmission. Since blocking activity inhibited phosphorylation of synaptic erbB4 (Fig. 1) but not extrasynaptic erbB4 (not shown), overexpression of erbB4 in the presence of activity blockers would shift erbB4 activation pattern away from the synapse. This may trap glutamate receptors away from the synapse (see below). An erbB4 kinase inhibitor, AG1478 (Fukazawa et al., 2003), also prevented the effects of erbB4 and occluded the effects of erbB4-KD (Fig. 2D, E). These data further indicate that normal spontaneous neuronal activity is required to activate erbB4, and activation of erbB4 kinase is necessary for its synaptic function.
ErbB4 regulates synaptic maturation
ErbB4 might affect synaptic transmission by controlling synaptic maturation that occurs during the period that constructs are expressed. To test this hypothesis, we examined the effects of erbB4 RNAi on miniature EPSC (mEPSC) of CA1 pyramidal cells. Normally there is a significant increase in mEPSC frequency (4 DIV: 3.3±0.7 per min, n=10 cells; 7 DIV: 17.5±4.5 per min, n=10 cells; P<0.05) and a small increase in mEPSC amplitude (4 DIV: 21.7±0.8 pA, n=300 events from 10 cells; 7 DIV: 25.8±0.5 pA, n=1330 events from 10 cells; P<0.05) during early development of CA3 – CA1 synapse (Fig. 3A, B, and C) (Barria and Malinow, 2005). The maturation process of excitatory synapse involves spontaneous activity-dependent plasticity (SAP) (Barria and Malinow, 2005; Kolleker et al., 2003; Zhu et al., 2002), which shares many properties with a more acute form of plasticity, long term potentiation (LTP), including its dependence on CaMK II and NMDAR activation (Barria and Malinow, 2005). ErbB4 RNAi largely arrested the increase in mEPSC frequency during development (4 DIV: 2.8±0.6 per min, n=8 cells; 7 DIV: 6.4±2.3 per min, n=10 cells; P>0.05; Fig. 3B, C) without significantly influencing mEPSC amplitude (4 DIV: 22.4±1.1 pA, n=212 events from 8 cells; 7 DIV: 24.9±0.8 pA, n=461 events from 10 cells; P>0.05 compared with control for both developmental stages; Fig. 3D). These data indicate that erbB4 mediated signaling is critical for normal synaptic maturation.
Figure 3. erbB4 regulates synaptic maturation.
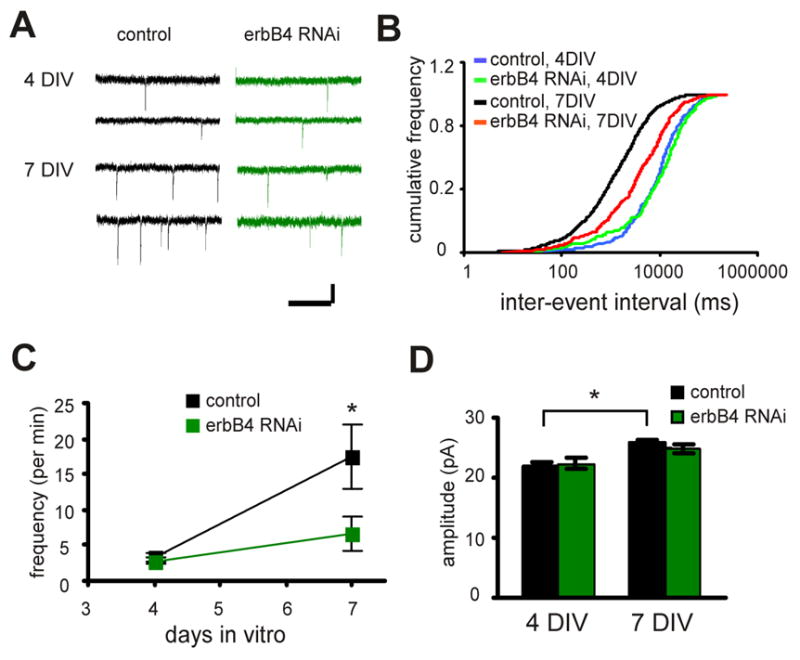
A. Representative traces of miniature events recorded from control neurons (left) or neurons expressing erbB4 RNAi (right) at different developmental stages (4 vs. 7 DIV). Scale bars: 1 s and 20 pA. B. Cumulative distribution of inter-event intervals for the miniature events recorded as in A. P<0.01 between control 4 DIV and 7 DIV, and between control and RNAi at 7 DIV, by K-S test). C. Quantification of the change in miniature event frequency during development for control neurons or neurons expressing erbB4 RNAi. D. Quantification of miniature event amplitude for control neurons and neurons expressing erbB4 RNAi at different development stages (4 vs. 7 DIV). *P<0.05.
ErbB4 is critical in maintaining spine morphology
Since erbB4 controls synaptic maturation, it is reasonable to speculate that erbB4 signaling also controls the size or number of dendritic spines, where excitatory synapses are placed (Harris and Kater, 1994). Indeed, erbB4 RNAi dramatically reduced spine density (control: 0.45±0.03 per μm, n=26 dendritic sections from 6 cells; erbB4 RNAi: 0.22±0.03 per μm, n=21 dendritic sections from 6 cells; P<0.01; Fig. 4A, C), and also significantly reduced spine size, although to a lesser extent (control: 1±0.03, n=540 spines from 6 cells; erbB4 RNAi: 0.8±0.04, n=221 spines from 6 cells; P<0.01; Fig. 4B). On the other hand, overexpression of erbB4 increased spine size (control: 1±0.08, n=303 spines from 4 cells; erbB4: 1.5±0.08, n=291 spines from 4 cells; P<0.01; Fig. 4D, E), but did not affect spine density (control: 0.6±0.05 per μm, n=9 dendritic sections from 4 cells; erbB4: 0.58±0.08 per μm, n=10 dendritic sections from 4 cells; P>0.05; Fig. 4F). This suggests that erbB4 activity is critical for the maintenance of existing spines, but is not sufficient for the formation of new spines. Interestingly, erbB4-KD only reduced spine size (erbB4-KD: 0.8±0.04, n=337 spines from 4 cells; P<0.05 compared with control; Fig. 4D, E), but did not change spine density (erbB4-KD: 0.56±0.03 per μm, n=9 dendritic sections from 4 cells; P>0.05 compared with control; Fig. 4F), an effect that is different from erbB4 RNAi (Fig. 4C). Since erbB4-KD still has other functional domains intact, such as the PDZ ligand domain (see below), it is likely that domains in erbB4 other than the kinase domain are also involved in maintaining spine structure.
Figure 4. erbB4 is critical for maintaining spine morphology.
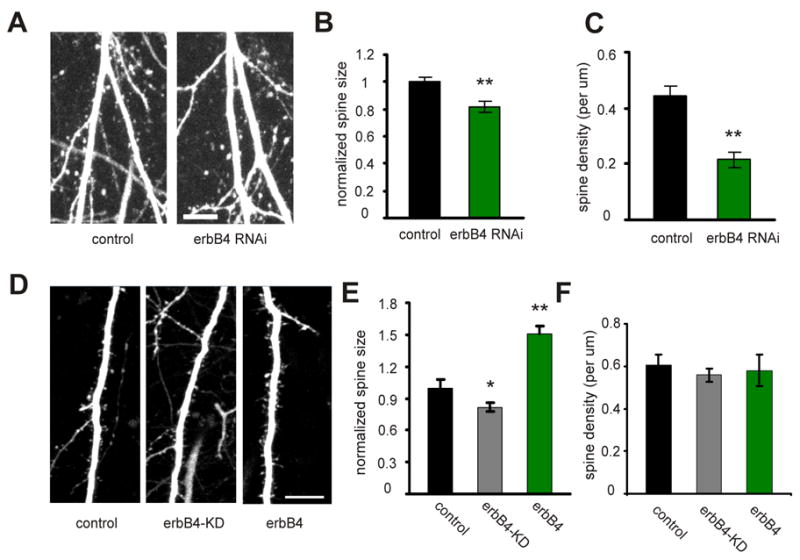
A. Representative images of apical dendrites from CA1 pyramidal neurons expressing GFP only (control) or erbB4 RNAi and GFP, acquired with TPLSM. B. Quantification of the spine size for control neurons and neurons expressing erbB4 RNAi. C. Quantification of the spine density for control neurons and neurons expressing erbB4 RNAi. D. Representative images of apical dendrites from CA1 pyramidal neurons expressing tomato dsRed only (control), tomato dsRed and SEP-erbB4-KD (erbB4-KD), or tomato dsRed and SEP-erbB4 (erbB4). Only red channel is shown for comparing spine morphology. E. Quantification of spine size for control, erbB4-KD, or erbB4 expressing neurons. F. Quantification of spine density for control, erbB4-KD, or erbB4 expressing neurons. *P<0.05; **P<0.01. Scale bar: 10 μm.
The PDZ interaction is important for erbB4 function
Previous studies have shown that the C-terminal end of erbB4 interacts with the PDZ domains of MAGUK proteins including PSD-95, and this interaction regulates the activation of erbB4 kinase (Huang et al., 2000; Xie et al., 2007). To test whether the erbB4 interaction with a MAGUK protein is important for its synaptic function, we expressed an erbB4 mutant, erbB4-ΔV, which has a single amino acid deletion at the very end of C-terminus that abolishes its PDZ interaction (Huang et al., 2000). ErbB4-ΔV lost the ability to enlarge spine size (control: 1±0.06, n=200 spines from 3 cells; erbB4-ΔV: 1±0.05; n=229 spines from 4 cells; p>0.05; Fig. 5A, B, and C) and potentiate synaptic transmission (control AMPA: 1±0.1, erbB4-ΔV AMPA: 1±0.1, n=13 pairs, p>0.05; control NMDA: 1±0.1, erbB4-ΔV NMDA: 0.86±0.16, n=12 pairs, p>0.05; Fig. 5D). Moreover, erbB4-ΔV is less enriched in the spine compared with wild-type erbB4 (erbB4: 47±6.3, n=212 spines from 3 cells; erbB4-ΔV: 31±4, n=229 spines from 4 cells; p<0.01; Fig. 5E) likely due to its inefficiency in activation (Huang et al., 2000), since activation of erbB4 is required for its spine enrichment (see Figure 1). However, it is also possible that interaction with a MAGUK protein directly controls erbB4 trafficking. We conclude that, besides the kinase domain, the PDZ ligand domain of erbB4 is also important for its proper synaptic localization and function.
Figure 5. The PDZ interaction is important of erbB4 function and localization.
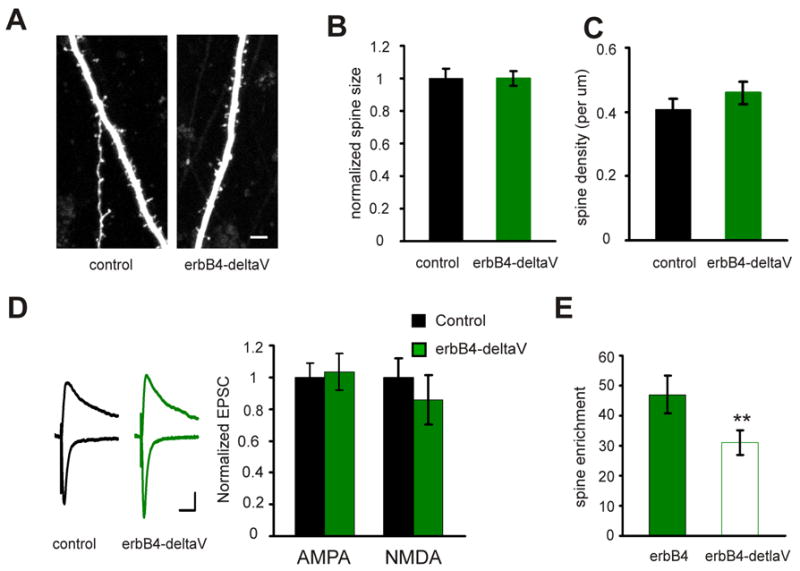
A. Representative images of apical dendrites from CA1 pyramidal neurons expressing tomato only (control) or SEP-erbB4-ΔV with tomato, acquired with TPLSM. Scale bar: 5 μm. B. Quantification of the spine size for control neurons and neurons expressing erbB4-ΔV. C. Quantification of the spine density for control neurons and neurons expressing erbB4-ΔV. D. Left: representative traces of EPSCs recorded simultaneously from a pair of neurons (black: control neuron; green: neuron overexpressing erbB4-ΔV). Scale bars: 50 ms and 20 pA. Right: quantification of the EPSCs mediated by both AMPAR and NMDAR from pairs of neurons. E. Quantification of spine enrichment of SEP-erbB4 and SEP-erbB4-ΔV.
ErbB4 mediates the function of NRG1
Although there is evidence showing that NRG1 is expressed in axons in the PNS (Fernandez et al., 2000; Taveggia et al., 2005), it is not clear if NRG1 is expressed in the presynaptic terminals of CNS glutamatergic synapse, mainly due to the lack of a high quality antibody to visualize the subcellular localization of NRG1. Moreover, since erbB4 has ligands other than NRG1, and erbB4 can form functional heteromers with other receptors such as erbB1 (Carpenter, 2003), a perturbation of erbB4 function may disrupt signaling pathways other than that mediated by NRG1. To directly address these questions we employed RNAi to knock down the expression of endogenous NRG1 in the presynaptic, CA3 pyramidal neurons. Three hairpin-like RNAs that target sequences that are shared by all major NRG1 isoforms, type I, II, and III, were expressed using Lenti viruses that co-express GFP or tDimer dsRed to serve as infection markers. The effect of the hairpins on NRG1 expression was confirmed in both hippocampal neurons and HEK293 cells transfected with different NRG1 isoforms (Supplemental Fig. 2A, B, C, and D). A hairpin that effectively reduced the expression of both NRG1 type I and III was used for subsequent experiments.
We reasoned that if the effects of erbB4 manipulation, as described above, are due to a disruption of presynaptic NRG1 mediated signaling, presynaptic knock down of NRG1 would occlude these effects. That is, a postsynaptic cell not expressing erbB4 RNAi would be depressed by presynaptic NRG1 RNAi to the same level as a postsynaptic cell expressing erbB4 RNAi. To test this, we infected CA3 area with a high titer Lenti virus expressing NRG1 RNAi together with tDimer. CA1 area was infected with low titer Lenti virus expressing erbB4 RNAi together with GFP. A stimulating electrode was positioned in the heavily infected (red) cell body area in CA3 (Fig. 6A) to activate CA3 axons expressing NRG1 RNAi. Simultaneous recordings of evoked EPSCs were made from paired non-infected and erbB4 NRAi-infected (green) pyramidal cells in the CA1 region (Fig. 6A). As expected for occlusion, the depressing effects of erbB4 RNAi on both AMPAR and NMDAR mediated transmission were abolished by NRG1 knock-down (AMPAR: control 1±0.2; erbB4 RNAi 1.1±0.1, n=18 paris, P>0.05; NMDAR: control 1±0.2; erbB4 RNAi 0.85±0.2, n=17 pairs, P>0.05; Fig. 6B, D). When presynaptic NRG1 RNAi was expressed together with a NRG1 construct with silent mutations that avoid RNAi targeting, the effects of postsynaptic expression of erbB4 RNAi on synaptic transmission were restored, suggesting that NRG1 RNAi is specific (Supplemental Fig. 2E). Furthermore, similar results were also obtained for erbB4-KD (AMPAR: control 1±0.1; erbB4-KD 0.87±0.1, n=14 pairs, P>0.05; NMDAR: control 1±0.2; erbB4-KD 0.99±0.2, n=12 pairs, P>0.05; Fig. 6C, E). These results indicate that postsynaptic erbB4 mediates the function of presynaptic NRG1 to control CA3 – CA1 synapses.
Figure 6. erbB4 mediates the function of presynaptic NRG1.
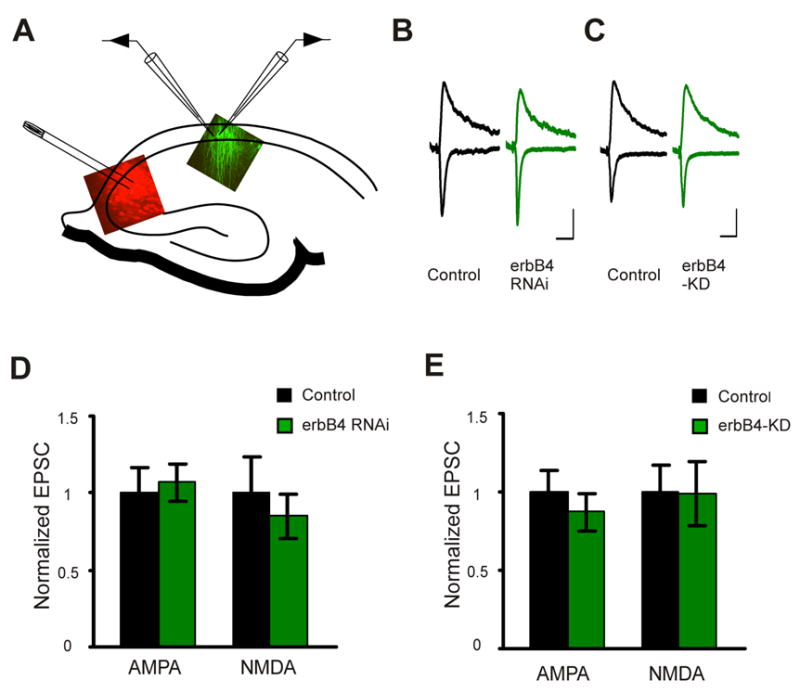
A. A diagram of the recording configuration for stimulating CA3 NRG1 RNAi expressing neurons (red), and recording a pair of CA1 neurons, which may include a non-infected neuron and an adjacent neuron expressing erbB4 RNAi or erbB4-KD (green). B and C. Representative traces from a pair of neurons recorded as in A. Scale bars: 50 ms and 20 pA. D. Quantification of the EPSCs mediated by both AMPAR and NMDAR from pairs of control and erbB4 RNAi expressing neurons recorded as in A. E. Quantification of the EPSCs mediated by both AMPAR and NMDAR from pairs of control and erbB4-KD expressing neurons recorded as in A.
NRG1/erbB4 signaling is critical for structural and functional synaptic plasticity
Since NRG1/erbB4 regulates spontaneous activity dependent plasticity (SAP), which shares mechanisms with LTP, we speculated that NRG1/erbB4 might also be important for LTP. To test this, either SEP-erbB4 or SEP-erbB4-KD was expressed in CA1 pyramidal cells, and the slices were incubated in elevated Mg2+ during the period of both erbB4 and erbB4-KD expression, which normalizes NMDA current between infected and non-infected cells (Fig. 2D, E). This will preclude the effect of NMDA receptor depression on synaptic plasticity. A chemical form of LTP (cLTP) (Kopec et al., 2006) combined with TPLSM was employed to track changes in individual spines induced by synaptic plasticity. Similar to what we have shown previously, cLTP, which induces transient synchronized neuronal bursting in organotypic slices (Kopec et al., 2006), induced a rapid and persistent increase in spine size (spine size at 5, 40, and 70 min was 1.7±0.1, 1.6±0.1, and 1.7±0.1, respectively, over base line; n=135 spines from 4 cells; P<0.01 for each time point; Fig. 7A). In addition, cLTP also induced a rapid and persistent increase in the amount of erbB4 receptors on the surface of spines (spine SEP-erbB4 at 5, 40, and 70 min was 1.36±0.1, 1.24±0.1, and 1.27±0.1, respectively, over base line; n=135 spines from 4 cells; P<0.01 for 5 min, and P<0.05 for 40 and 70 min time points; Fig. 7A), further supporting the finding that neuronal activity recruits erbB4 into the synapse. The increase of erbB4 on the spine surface is unlikely to be a passive recruitment due to the increase in spine size, as other surface proteins, such as NMDARs, do not increase in spines during cLTP (Kopec et al., 2006). On the other hand, SEP-erbB4-KD largely prevented the spine size increase induced by cLTP (spine size at 5, 40, and 70 min was 1.2±0.1, 1±0.1, and 1.1±0.1, respectively, over base line; n=121 spines from 4 cells; P<0.01 compared with SEP-erbB4 for each time point; Fig. 7B), and cLTP did not increase the content of SEP-erbB4-KD on spine surface (spine SEP-erbB4-KD at 5, 40, and 70 min was 1±0.04, 0.87±0.04, and 0.98±0.1, respectively, over base line; n=135 spines from 4 cells; P>0.05; Fig. 7B). This suggests that the normal NRG1/erbB4 signaling is required for cLTP induction and erbB4 trafficking.
Figure 7. NRG1/erbB4 signaling regulates structural and functional plasticity.
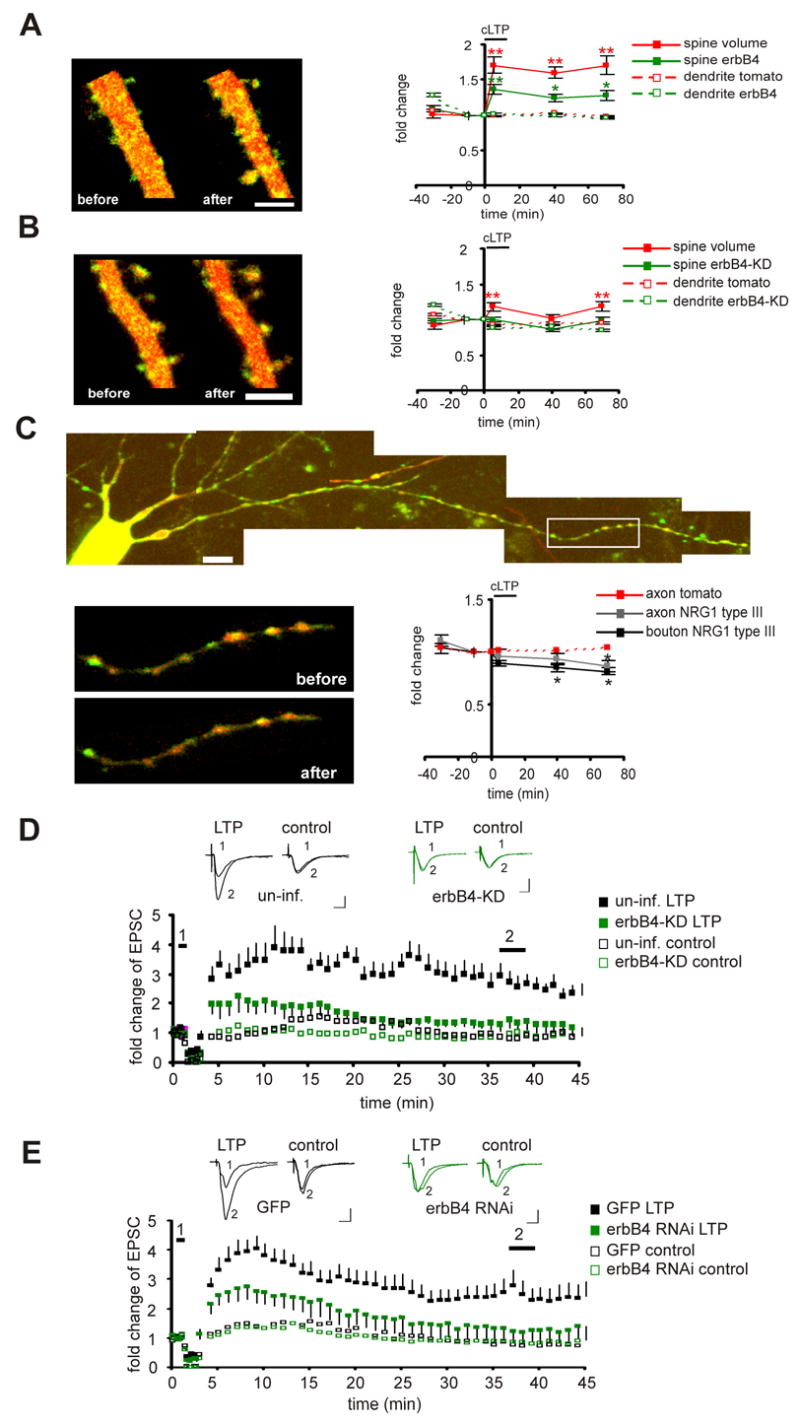
A. Left: images of a CA1 pyramidal cell expressing tomato dsRed and SEP-erbB4 taken at −30 and +40 min relative to cLTP induction. Scale bar: 5 μm. Right: Quantification of the integrated red (volume) and green (erbB4) fluorescence for spines, as well as the mean red (tomato dsRed) and mean green (erbB4) value for dendrites at each time point during the cLTP experiments. Each region of interest is normalized to its value at the −10 min time point. The black bar denotes cLTP induction. B: same as A, except SEP-erbB4-KD was expressed instead of SEP-erbB4. C: images of a CA3 pyramidal cell expressing tDimer dsRed and SEP-NRG1 type III. Scale bar: 10 μm. The boxed axonal region was followed before, during, and after cLTP induction. Higher magnification pictures of this region, taken at −30 and +40 min relative to cLTP induction, are shown at below. Quantification of the green (NRG1) over red (tDimer) fluorescence at boutons or axonal regions at each time point during the cLTP experiments is shown at the right. These values are measurements of relative NRG1 density at each ROI. Each region of interest is normalized to its value at the −10 min time point. The black bar denotes cLTP induction. D. Top: representative traces of AMPAR mediated EPSCs from uninfected neurons (left) or neurons expressing erbB4-kd (right). Traces are averaged for time points before (1) and after (2) LTP induction for the induced pathway (LTP) or control pathway (control). Scale bars: 50 ms and 20 pA. Bottom: Normalized amplitudes of AMPAR mediated responses before and after delivery of LTP-induction protocol (arrow). E. same as D, except that comparison was made between neurons expressing GFP and neurons expressing erbB4-RNAi. *P<0.05; **P< 0.01 relative to baseline.
Activity-dependent activation of erbB4 might result from the activity-dependent processing or release of NRG1 from the presynaptic termini, since it has been shown that NRG1 type I expression, processing, or release is regulated by neuronal activity, including LTP like stimulation (Eilam et al., 1998; Han and Fischbach, 1999; Ozaki et al., 2004). Less is known about the activity-dependent regulation of NRG1 type III, although its processing has also been shown to be activity dependent (Bao et al., 2003). To directly visualize NRG1 type III, we tagged NRG1 type III with SEP in the extracellular region (Wang et al., 2001). After infection of pyramidal neurons with a double-promoter Sindbis virus expressing SEP-NRG1 type III together with tDimer as a morphological marker, the subcellular distribution of SEP-NRG1 type III was examined with TPLSM. SEP-NRG1 type III was readily detectable on the surface of axons, especially on bouton-like structures (Fig. 7C). To test if the distribution of NRG1 is regulated by neuronal activity, time-lapse TPLSM was used to monitor SEP-NRG1 type III before, during, and after cLTP. We quantified the amount of NRG1 on the boutons or axons as the ratio of the mean green signal (SEP-NRG1) to the mean red signal (tDimer), which serves as a relative density of NRG1 on those structures (Kopec et al., 2006). The cLTP significantly reduced the amount of NRG1 type III on the surface of axon boutons (bouton NRG1 at 40 and 70 min were 0.85±0.04 and 0.81±0.03 over base line; n=32 boutons from 4 cells, P<0.05; Fig. 7C), and also reduced the amount on other axonal regions (axonal NRG1 at 40 and 70 min were 0.93±0.05 and 0.87±0.05 over base line; n=32 regions from 4 cells, P<0.05; Fig. 7C). The decrease in NRG1 density is not due to a change in bouton size, since boutons did not enlarge after cLTP (Fig. 7C). The red signal on the axons was stable during the period of experiments, suggesting the cell condition was good and photo bleaching was minimal. The reduction in the surface NRG1 type III may result from the processing and subsequent endocytosis of the surface NRG1, suggesting that the EGF domain on a subpopulation of NRG1 type III was briefly exposed and presented to its receptors during cLTP.
To test further whether NRG1/erbB4 is also required for the conventional, electrically induced LTP, we compared LTP in CA1 control pyramidal cells or cells expressing erbB4-KD. ErbB4-KD significantly reduced LTP (control: n=7; erbB4-KD: n=7; P<0.05 measured between 35 to 45 min; Fig. 7D). Similarly, cells expressing erbB4 RNAi also had reduced LTP compared with cells expressing only GFP (GFP: n=16 reduced to 13; erbB4 RNAi: n=16; P<0.05 measured between 35 to 45 min; Fig. 7E). . We conclude that the normal NRG1/erbB4 signaling is necessary for both structural and functional LTP.
NRG1/erbB4 regulates synaptic function and structure by stabilizing AMPA receptors
A previous study from our laboratory showed that the synaptic depression and spine loss resulting from increased levels of beta amyloid can be blocked by stabilizing AMPA receptors in the synapse (Hsieh et al., 2006). To test whether stabilizing AMPA receptors could prevent the defect in synaptic transmission and spine morphology caused by NRG1/erbB4 loss of function, we examined the effects of a peptide derived from the AMPA receptor gluR2 C-terminus. This peptide, gluR2-3Y, has been shown to stabilize AMPA receptors in the synapse (Ahmadian et al., 2004). Strikingly, incubation of slices with gluR2-3Y abolished the effects of erbB4 RNAi on both synaptic transmission (AMPA control: 1±0.1; AMPA erbB4 RNAi: 1±0.1, n=16 pairs, p>0.05; NMDA control: 1±0.2; NMDA erbB4 RNAi: 0.97±0.2, n=14 pairs, p>0.05; Fig. 8A) and spine morphology (spine size: control 1±0.05, n=302 spines from 4 cells; erbB4 RNAi: 0.9±0.05; n=317 spines from 4 cells; p>0.05; spine density: control 0.54±0.04, n=12 dendritic sections from 4 cells; erbB4 RNAi 0.52±0.04, n=13 dendritic sections from 4 cells; p>0.05; Fig. 8C, D, and E). Incubation of slices with a mutant version of the peptide, gluR2-3A, which has no effects on AMPA receptor synaptic stabilization (Ahmadian et al., 2004), had no effects on erbB4 RNAi induced synaptic depression (AMPA control: 1±0.1; AMPA erbB4 RNAi: 0.68±0.1, n=16 pairs, p<0.01; NMDA control: 1±0.2; NMDA erbB4 RNAi: 0.7±0.1, n=14 pairs, p<0.05; Fig. 8B). These results indicate that NRG1/erbB4 signaling controls synaptic function and structure by regulating AMPA receptor stabilization in the synapse.
Figure 8. erbB4 regulates synaptic function and structure by stabilizing AMPARs.
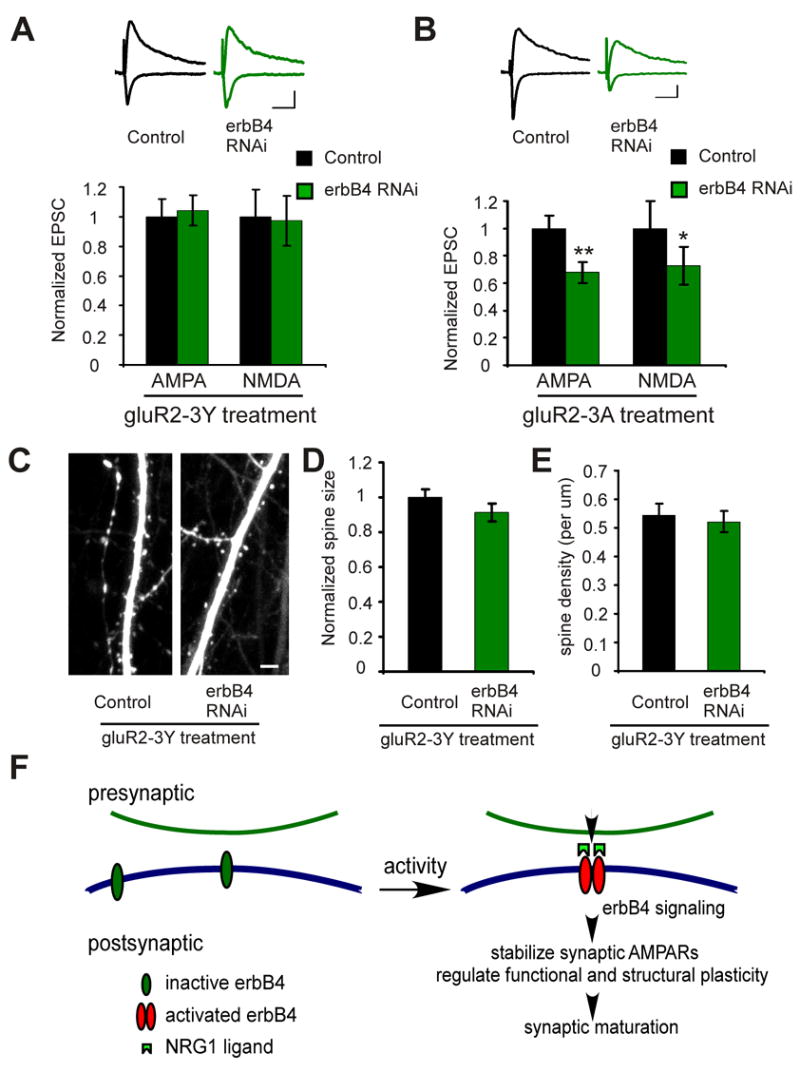
A. Neurons were treated with the gluR2-3Y peptide. Upper: representative traces of EPSCs recorded simultaneously from a pair of neurons (black: control neuron; green: neuron expressing erbB4 RNAi). Scale bars: 50 ms and 20 pA. Lower: quantification of the EPSCs mediated by both AMPAR and NMDAR from pairs of neurons. B. Same as A, except that the neurons were treated with the gluR2-3A peptide. C. Representative images of apical dendrites from CA1 pyramidal neurons treated with the gluR2-3Y peptide. Neurons express either GFP only (control) or erbB4 RNAi. D and E. Quantification of the spine size (D) or density (E) for control neurons and neurons expressing erbB4 RNAi. Scale bar: 5 μm. F. A schematic model for NRG1/erbB4 function in the synapse. Synaptic activity, including NMDAR function, leads to activation of postsynaptic erbB4, which stabilizes synaptic AMPARs, permitting structural and functional maturation.
Discussion
A prominent hypothesis regarding the underlying pathophysiology of schizophrenia has proposed that hypofunction of the glutamatergic system plays a causal role (Coyle and Tsai, 2004; Harrison, 2004; Harrison et al., 2003; Harrison and Weinberger, 2005). In humans, reducing glutamatergic transmission can mimic (Pietraszek, 2003), while enhancing gluamatergic transmission can alleviate (Javitt, 2004) schizophrenic symptoms. Patients with the disease show decreased excitatory synaptic function in hipocampal and cortical regions (Abbott and Bustillo, 2006; Bressan and Pilowsky, 2000; Pilowsky et al., 2006).
Another feature thought to be important in the disease is its neurodevelopmental nature. For example, animals with lesions that disrupt the excitatory connections between hippocampus and the frontal cortex early in development show schizophrenic-like symptoms later in life (Bertolino et al., 1997; Daenen et al., 2003; Lipska, 2004; Van den Buuse et al., 2003). Human studies also suggest that pre- or perinatal brain lesions may lead to schizophrenia by disrupting early brain maturation processes including those in the hippocampus (Pantelis et al., 2005; Rehn, 2005). It is thus believed that perturbation of circuitry formation early in life can predispose to the disease.
Schizophrenia is also a genetic disease. With the advent of molecular genetics a number of genes have been linked to schizophrenia (Harrison and Weinberger, 2005). The gene with most supportive evidence is nrg1 (Harrison and Weinberger, 2005; Norton et al., 2006b).
Despite these advances in the field, there has not been a mechanism that can link genetic perturbation of NRG1 signaling to glutamatergic hypofunction or developmental abnormalities.
In this study, we examined the function of NRG1/erbB4 signaling in the excitatory CA3 – CA1 synapse in the hippocampus. Our data support a model in which synaptic activity leads to the activation of NRG1/erbB4 signaling pathway, which recruits or stabilizes erbB4 in the synapse in a manner that depends on erbB4 PDZ interaction. Activity dependent NRG1/erbB4 activation stabilizes synaptic AMPA receptors and thus permits synaptic plasticity and synaptic maturation (Fig. 8F). Interruption of NRG1/erbB4 signaling causes destabilization of synaptic AMPARs and spine structure, leading to impairment in plasticity and eventually loss of spines and NMDARs. These results therefore provide a direct link between NRG1 and glutamatergic function. Decreased NRG1/erbB4 signaling (which could be longer term but more modest than the knockdown or dominant negative perturbations in this study) would likely have manifestations on excitatory circuit development by inhibiting normal functional and structural synaptic maturation. Thus, our study provides a link between nrg1 and the “glutamatergic hypofunction” hypothesis as well as with the view that development of early circuitry is an important underlying factor in schizophrenia.
We found an interesting relationship between NRG1/ErbB4 signaling and synaptic activity. Synaptic activity is required for NRG1/ErbB4 signaling and NRG1/ErbB4 signaling enhances synaptic function. Such positive feedback relationships could lead to a switch-like behavior, where too little activity would lead to a weakening of the synapse and achieving a threshold level of activity would lead to its saturation. Since synapses appear to display intermediate strengths, there are likely to be other signaling systems that strongly modulate this switch-like function. Aberrations of these other modulatory signaling systems would likely abrogate NRG1/erbB4 signaling and could contribute to schizophrenia. Our finding that NRG1/ErbB4 signaling depends on synaptic activity indicates that the well-known impact of experience on synaptic structure and function is likely to act, at least in part, through this signaling pathway.
Several recent studies have examined the acute effects of NRG1 on synaptic function in the CNS. NRG1 application has been shown to inhibit synaptic plasticity (Huang et al., 2000; Kwon et al., 2005; Ma et al., 2003) and to reduce NMDA receptor activity (Gu et al., 2005) or synaptic transmission (Roysommuti et al., 2003). However, the interpretation of these data is complicated since these studies employed tonic application of a functional domain (such as the EGF domain) of certain isoforms of recombinant NRG1 (Gu et al., 2005; Huang et al., 2000; Kwon et al., 2005; Ma et al., 2003; Roysommuti et al., 2003), and little is known about the physiological concentration, activity duration, location of activity, specific NRG1 isoforms involved, as well as different erbB receptors being activated by such application. The function of endogenous NRG1 in the synapse may thus be different. Indeed, NRG1 deficient mice have reduced NMDA receptor activity compared with wild-type (Stefansson et al., 2002), rather than increased NMDA receptor activity as would be predicted from the acute application studies.
In conclusion, our study indicates a mechanism by which core features of schizophrenia, including genetic deficits, developmental abnormalities, and glutamatergic hypofunction can be linked together. The direct and chronic perturbation of NRG1/erbB4 signaling, such as mutations in NRG1 or erbB4 genes that occur in some schizophrenia patients (Walss-Bass et al., 2006), would lead to the abnormal development and hypofunction of glutamatergic synapse and circuitry. Supporting this view, a decrease in spine density and other markers for excitatory synapses has been reported in the brain of schizophrenia patients (Eastwood, 2004; Harrison et al., 2003; Kristiansen et al., 2006; Lewis et al., 2003). Mutations in NRG1 or erbB4 genes likely account for only a fraction of schizophrenia cases (Harrison and Weinberger, 2005). For other patients there may be defects in other signaling or structural components producing hypofunction in the glutamatergic pathway, and a compensatory increase in NRG1/erbB4 activity or expression level might be expected. Indeed, an increase in the expression level of NRG1 isoforms (Hashimoto et al., 2004; Law et al., 2006; Petryshen et al., 2005) or erbB4 (Silberberg et al., 2006), as well as an increase in the activity of the NRG1/erbB4 signaling pathway (Hahn et al., 2006), have been found in some schizophrenia patients.
Methods
Biochemistry on PSD fraction, immunoprecipitation and Western Blotting
See Supplemeental Data.
Plasmid construction
The super-ecliptic pHluorin (SEP) coding sequence (G. Miesenbock, personal communication) was inserted after the predicted signal peptide cleavage site of human erbB4 CYT-2 isoform. The resultant product was inserted into the mammalian expression vector pCDNA3 (Invitrogen). The kinase-inactive mutant erbB4-KD contains a point mutation (K751M) in the kinase domain. The red fluorescent proteins (tDimer or tomato dsRed), fast maturing obligate dimer versions of dsRed (provided by R. Tsien, University of California San Diego, La Jolla, CA), were inserted into either pCI (Promega) or pCDNA3. To generate SEP-NRG1, SEP was inserted into an extracellular region of either NRG1 type I or type III. This region has been previously used to insert a HA or GFP tag (Ozaki et al., 2004; Wang et al., 2001). To facilitate imaging of SEP-NRG1, the SEP-NRG1 type III was cloned into a double-promoter Sindbis viral vector that express both SEP-NRG1 and tDimer. The double-promoter Sindbis vector was a gift from Dr. H. Nawa.
Short hairpin RNA based RNA interference for erbB4 and NRG1
To suppress the expression of endogenous erbB4 or NRG1, short haipin RNAs specifically against rat erbB4 or NRG1 were stably expressed using a strategy developed in the Hannon lab (Paddison et al., 2002). To suppress erbB4 or NRG1 expression in neurons, Lenti virus was used to express both short hairpin RNAs, which is driven by the U6 promoter (Paddison et al., 2002), and GFP or tDimer, which is driven by the Ubiquitin or Synapsin promoter, to visualize the infected neurons. The Lenti viral construct, which is similar to what has been described previously (Dittgen et al., 2004), was a gift from Dr. G. Hannon. The target sequences of three short hairpin RNAs for erbB4 are: 5′GCCGTTTATGTCAGAAGGAAG3′; 5′CCAGACTACCTGCAGGAATAC3′; 5′GCCCGCAATGTGTTGGTGAAA3′. The target sequences of three short hairpin RNAs for NRG1 are: 5′ACTCAGAAAGTGAGACAGAAG3′; 5′GGCCAGGCTGTCTAGTGTAAT3′; 5′ACGGAGAGCGTCATTTCAGAA3′. These sequences are present in all three major isoforms of NRG1: NRG1 type I, type II, and type III.
Slice culture, transfection, and other treatments
Organotypic hippocampal slices were prepared from postnatal day 6 or 7 rats (Stoppini et al., 1991), transfected after 4–6 d in culture, and used for electrophysiology or imaging experiments 2–3 d after transfection. Coexpression of two constructs was achieved using biolistics transfection (Arnold et al., 1994). For experiments using Lenti virus infection, slices were infected after 1 d in culture, and used 4–7 days after infection for electrophysiology, or 7 days after infection for imaging experiments.
For treatment with gluR2-3Y or 3A peptides, slices were first infected with Lenti virus at day 0. At day 4 the peptides were added to the medium at a concentration of 5 μm. The peptides were replenished every 24 hours. Recordings were made on day 7. The sequences of gluR2-3Y and 3A are the same as previously reported (Ahmadian et al., 2004). A TAT sequence was fused to peptides to aid delivery into the cell. A dansyl group was also included for visualization of the peptides. Accumulation of the peptides in neurons was readily detectable by two-photon laser scanning microscope at a wavelength of 720 nm (data not shown).
Electrophysiology
See Supplemental Data for details.
Two-photon laser-scanning microscopy and cLTP induction, image display, and quantitative image analysis
Same as that in (Kopec et al., 2006), or see Supplemental Data for details.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R.M., L.M.) and the Canadian Institute of Health Research (B.L.; Postdoctoral Fellowship). We thank Nancy Dawkins for preparation of hippocampal slice cultures; Yu Fu for making the NRG1 expressing constructs in Sindbis vector; Dr. Charles D. Kopec for programming image analysis software; Barry Burbach, Peter O’Brien, and Dr. Svoboda for assistance on the microscope; Drs. Tsien, Miesenbock, Hannon, and Nawa for supplying reagents; Dr. S. Muthuswamy and members of the Malinow lab for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19:135–139. doi: 10.1097/01.yco.0000214337.29378.cd. [DOI] [PubMed] [Google Scholar]
- Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. Embo J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Arnold D, Feng L, Kim J, Heintz N. A strategy for the analysis of gene expression during neural development. Proc Natl Acad Sci U S A. 1994;91:9970–9974. doi: 10.1073/pnas.91.21.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex. 1997;7:740–748. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Pilowsky LS. Imaging the glutamatergic system in vivo--relevance to schizophrenia. Eur J Nucl Med. 2000;27:1723–1731. doi: 10.1007/s002590000372. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Calaora V, Rogister B, Bismuth K, Murray K, Brandt H, Leprince P, Marchionni M, Dubois-Dalcq M. Neuregulin signaling regulates neural precursor growth and the generation of oligodendrocytes in vitro. J Neurosci. 2001;21:4740–4751. doi: 10.1523/JNEUROSCI.21-13-04740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL. Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:42–54. doi: 10.1093/jnen/62.1.42. [DOI] [PubMed] [Google Scholar]
- Chu GC, Moscoso LM, Sliwkowski MX, Merlie JP. Regulation of the acetylcholine receptor epsilon subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Van Der Heyden JA, Kruse CG, Van Ree JM. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol. 2003;13:187–197. doi: 10.1016/s0924-977x(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL. The synaptic pathology of schizophrenia: is aberrant neurodevelopment and plasticity to blame? Int Rev Neurobiol. 2004;59:47–72. doi: 10.1016/S0074-7742(04)59003-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci U S A. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Escher P, Lacazette E, Courtet M, Blindenbacher A, Landmann L, Bezakova G, Lloyd KC, Mueller U, Brenner HR. Synapses form in skeletal muscles lacking neuregulin receptors. Science. 2005;308:1920–1923. doi: 10.1126/science.1108258. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000;28:81–90. doi: 10.1016/s0896-6273(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA> receptor hypofunction in schizophrenia. Nat Med. 2006 doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Fischbach GD. Processing of ARIA and release from isolated nerve terminals. Philos Trans R Soc Lond B Biol Sci. 1999;354:411–416. doi: 10.1098/rstb.1999.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and Schizophrenia: Genetics, Gene Expression, and Neurobiology. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann N Y Acad Sci. 2003;1003:94–101. doi: 10.1196/annals.1300.006. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry . 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry . 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- Kolleker A, Zhu JJ, Schupp BJ, Qin Y, Mack V, Borchardt T, Kohr G, Malinow R, Seeburg PH, Osten P. Glutamatergic plasticity by synaptic delivery of GluR-B(long)-containing AMPA receptors. Neuron. 2003;40:1199–1212. doi: 10.1016/s0896-6273(03)00722-0. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry . 2006;11:737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Glantz LA, Pierri JN, Sweet RA. Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann N Y Acad Sci. 2003;1003:102–112. doi: 10.1196/annals.1300.007. [DOI] [PubMed] [Google Scholar]
- Lin W, Sanchez HB, Deerinck T, Morris JK, Ellisman M, Lee KF. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc Natl Acad Sci U S A. 2000;97:1299–1304. doi: 10.1073/pnas.97.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. J Psychiatry Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Lu B, Xiong WC, Salter MW, Weinberg RJ, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006a;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Norton N, Williams HJ, Owen MJ. An update on the genetics of schizophrenia. Curr Opin Psychiatry. 2006b;19:158–164. doi: 10.1097/01.yco.0000214341.52249.59. [DOI] [PubMed] [Google Scholar]
- Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Tohyama K, Kishida H, Buonanno A, Yano R, Hashikawa T. Roles of neuregulin in synaptogenesis between mossy fibers and cerebellar granule cells. J Neurosci Res. 2000;59:612–623. doi: 10.1002/(SICI)1097-4547(20000301)59:5<612::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, et al. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry . 2005;10:366–374. 328. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]
- Pietraszek M. Significance of dysfunctional glutamatergic transmission for the development of psychotic symptoms. Pol J Pharmacol. 2003;55:133–154. [PubMed] [Google Scholar]
- Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- Rehn A, Alexandra E|Rees SM, Sandra M. Investigating the neurodevelopmental hypothesis of schizophrenia. Clinical and experimental pharmacology & physiology. 2005;32:687–696. doi: 10.1111/j.1440-1681.2005.04257.x. [DOI] [PubMed] [Google Scholar]
- Rieff HI, Corfas G. ErbB receptor signalling regulates dendrite formation in mouse cerebellar granule cells in vivo. Eur J Neurosci. 2006;23:2225–2229. doi: 10.1111/j.1460-9568.2006.04727.x. [DOI] [PubMed] [Google Scholar]
- Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. Neuregulin induces GABA(A) receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Carroll SL, Wyss JM. Neuregulin-1beta modulates in vivo entorhinal-hippocampal synaptic transmission in adult rats. Neuroscience. 2003;121:779–785. doi: 10.1016/s0306-4522(03)00503-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, de Kerchove d’Exaerde A, Changeux JP. Targeting transcription to the neuromuscular synapse. Neuron. 2001;31:15–22. doi: 10.1016/s0896-6273(01)00353-1. [DOI] [PubMed] [Google Scholar]
- Schmucker J, Ader M, Brockschnieder D, Brodarac A, Bartsch U, Riethmacher D. erbB3 is dispensable for oligodendrocyte development in vitro and in vivo. Glia. 2003;44:67–75. doi: 10.1002/glia.10275. [DOI] [PubMed] [Google Scholar]
- Scolnick EM, Petryshen T, Sklar P. Schizophrenia: do the genetics and neurobiology of neuregulin provide a pathogenesis model? Harv Rev Psychiatry. 2006;14:64–77. doi: 10.1080/10673220600642960. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann N Y Acad Sci. 2004;1025:84–91. doi: 10.1196/annals.1316.011. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Fischbach GD. Purification and characterization of a polypeptide from chick brain that promotes the accumulation of acetylcholine receptors in chick myotubes. J Cell Biol. 1986;103:493–507. doi: 10.1083/jcb.103.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Buuse M, Garner B, Koch M. Neurodevelopmental animal models of schizophrenia: effects on prepulse inhibition. Curr Mol Med. 2003;3:459–471. doi: 10.2174/1566524033479627. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Corfas G, Li Y, Fischbach GD, Stefansson K. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc Natl Acad Sci U S A. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian T, Fischbach G, Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc Natl Acad Sci U S A. 1999;96:731–735. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walss-Bass C, Liu W, Lew DF, Villegas R, Montero P, Dassori A, Leach RJ, Almasy L, Escamilla M, Raventos H. A novel missense mutation in the transmembrane domain of neuregulin 1 is associated with schizophrenia. Biol Psychiatry. 2006;60:548–553. doi: 10.1016/j.biopsych.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- Xie F, Padival M, Siegel RE. Association of PSD-95 with ErbB4 facilitates neuregulin signaling in cerebellar granule neurons in culture. J Neurochem. 2007;100:62–72. doi: 10.1111/j.1471-4159.2006.04182.x. [DOI] [PubMed] [Google Scholar]
- Xie F, Raetzman LT, Siegel RE. Neuregulin induces GABAA receptor beta2 subunit expression in cultured rat cerebellar granule neurons by activating multiple signaling pathways. J Neurochem. 2004;90:1521–1529. doi: 10.1111/j.1471-4159.2004.02685.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- Yang XL, Huang YZ, Xiong WC, Mei L. Neuregulin-induced expression of the acetylcholine receptor requires endocytosis of ErbB receptors. Mol Cell Neurosci. 2005;28:335–346. doi: 10.1016/j.mcn.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


