Abstract
Objective
We have evaluated T cell reconstitution and reactivity in patients receiving non-myeloablative haploidentical hematopoietic cell transplantation (HCT) protocols involving an anti-CD2 monoclonal antibody (MEDI 507) to treat chemorefractory hematopoietic malignancies.
Methods
Three cohorts of 4 patients each and 1 cohort of 6 patients received one of four Medi-507-based regimens, all of which included cyclophosphamide, thymic irradiation and a short post-transplant course of cyclosporine.
Results
Following marked T cell depletion, initially recovering CD4 and CD8 T cells were mainly memory-type cells. A high percentage of CD4 T cells expressed high levels of CD25 in recipients of all protocols except the only protocol to include fludarabine, early post-HCT. CD25 expression varied inversely with T cell concentrations in blood. CD25high CD4 T cells expressed Foxp3 and CTLA4, indicating that they were regulatory T cells (Treg).
Conclusions
Fludarabine treatment prevents Treg enrichment after haploidentical non myeloablative stem cell transplantation, presumably by depleting recipient Tregs. In vitro analyses of allorecognition were consistent with a cytokine-mediated rejection process in one case and in another provided proof of principle that mixed chimerism achieved without GVHD induces donor- and recipient-specific tolerance. More reliable achievement of this outcome could provide a promising strategy for organ allograft tolerance induction.
Introduction
Severe graft-versus-host disease (GVHD) and graft rejection are more common following haploidentical than HLA-matched related donor hematopoietic cell transplantation (HCT) HCT [1-8]. Due to the shortage of HLA-matched related donors and the difficulties associated with transplantation from unrelated donors, strategies are needed to improve the outcomes of transplants from haploidentical related donors. Besides significantly expanding the available donor pool, a potential major advantage of successful allogeneic transplantation across HLA barriers would be improved anti-tumor effects, owing to an enhanced GVH alloresponse that also eliminates malignant cells [1,2,9-12]. Following myeloablative conditioning and high dose vigorously T cell-depleted peripheral blood stem cell (PBSC) transplantation, sustained alloengraftment has been reliably achieved [13]. The major obstacles to more successful application of this strategy have included prolonged immunodeficiency and associated infection risk, as well as toxicities associated with myeloablative conditioning regimens.
Non-myeloablative haploidentical HCT might be associated with reduced transplant-related morbidity and mortality compared to myeloablative approaches. We have developed an approach involving non-myeloablative conditioning in an effort to achieve initial mixed chimerism without a GVH reaction by using in vivo T cell-depleting mAbs, most recently along with ex vivo T cell depletion [5,9]. Based on data in mice [2-4], we hypothesize that potent GVT effects and restoration of immune competence can be achieved following donor leukocyte infusion (DLI), without the complication of GVHD. In our animal model, administration of DLI after sufficient time has passed for conditioning-induced inflammation to subside allows the GVH alloresponse of DLI to be confined to the lymphohematopoietic tissues. The activated GVH-reactive T cells do not traffic to the epithelial GVHD target tissues under such conditions [14], allowing GVL to be achieved without GVHD [4,15].
Our efforts to apply this approach clinically to haploidentical transplantation involve the use of cyclophosphamide, thymic irradiation and MEDI-507, a humanized anti-CD2 mAb, to deplete T cells in vivo, followed by a short course of cyclosporine [9]. Eighteen patients with chemorefractory hematologic malignancies received one of four MEDI-507-based non-myeloablative preparative regimens for haploidentical HCT. The clinical outcomes of the first twelve patients have been described previously [9]. Here, we report on the post-transplant T cell reconstitution and alloreactivity of these patients. Our results provide insights into the ability to achieve tolerance across HLA barriers via mixed chimerism.
Materials and Methods
Conditioning and Transplantation
Eighteen patients with advanced hematologic malignancies received non-myeloablative haploidentical HCT with Medi-507 under IRB-approved protocols at Massachusetts General Hospital (Table I). HLA-A, HLA-B and HLA-C were typed at low resolution, and HLA-DRB1 and DQB1 were typed at high resolution using standard polymerase reaction (PCR)-based methods. Conditioning involved cyclophosphamide, 150 mg/kg (50 mg/kg on Days −5, −4 and −3), and thymic irradiation (700cGy) on Day −1 (n=10) in patients who had not received previous mediastinal radiation therapy. Cyclosporine was begun on Day −1 at a dose of 5mg/kg IV. The dose was decreased to 3 mg/kg on Day +4, and tapered and discontinued by approximately Day +35 post-transplant in the absence of GVHD. Patients with mixed chimerism and no evidence of GVHD or increasing T cell chimerism were eligible to receive a “prophylactic” DLI two weeks after CYA discontinuation, intended to convert their mixed chimerism to full donor hematopoiesis. Patients could also receive “therapeutic” DLI for disease recurrence or progression (Table I).
Table 1. Patient characteristics, outcome, chimerism and T cell reconstitution after haploidentical Hematopoietic Cell Transplantation.
| Disease | HLA MM GVH;HVG | Day to T cell >100/μl | Max chim T cell % (day) | Max chim Myeloid % (day) | aGVHD grade | p/t DLI (day) | Graft loss (day) | Response | |
|---|---|---|---|---|---|---|---|---|---|
| Protocol A | |||||||||
| Patient A1 | NHL | 3/10;3/10 | 135 | 16.9 (7) | 43.3 (7) | None | tDLI (35,59) | Yes (14) | PD |
| Patient A2 | NHL | 4/10;3/10 | 100 | 86.5 (all) (14) | NA | None | pDLI (79) | Yes (21) | PD |
| Patient A3 | NHL | 3/10;4/10 | 111 | 74 (13) | 37.8 (13) | None | pDLI (62) | Yes (76) | CR |
| Patient A4 | AML | 4/10;5/10 | NA | 60 (all) (7) | NA | None | tDLI (35) | Yes (47) | PD |
| Protocol B | |||||||||
| Patient B1 | HD/NHL | 5/10;1/10 | 15 | 100 (42) | 100 (42) | IV | none | No | CR |
| Patient B2 | NHL | 3/10;2/10 | 91 | 100 (56) | 100 (27) | II | none | No | CR |
| Patient B3 | HD | 4/10;4/10 | 22 | 10.9 (7) | 100 (7) | None | pDLI (43) | Yes (41) | PD |
| Patient B4 | NHL | 3/10;5/10 | NA | NA | NA | NA | none | NA | NA |
| Protocol C | |||||||||
| Patient C1 | NHL | 2/10;3/10 | 28 | 20 (28) | NA | None | pDLI (52,80,120) | Yes (77) | CR |
| Patient C2 | NHL | 4/10;3/10 | 58 | 100 (90) | 99.6 (20) | None | tDLI (66) | No | PD |
| Patient C3 | NHL | 4/10;4/10 | 57 | 92.3 (79) | 92.3 (79) | II post DLI | tDLI (51) | No | PD |
| Patient C4 | NHL | 3/10;5;10 | 47 | 14.2 (47) | 14.2 (47) | None | tDLI (96,120) | Yes (74) | PR |
| Protocol D | |||||||||
| Patient D1 | NHL | 2/10;2/10 | 7 | 87 (175) | 100 (125) | I | none | No | CR |
| Patient D2 | HD | 2/10;2/10 | 14 | 97 (64) | 90 (64) | II | none | No | PD |
| Patient D3 | NHL | 2/10;4/10 | 49 | 93 (91) | 98 (91) | II post DLI | tDLI (48) | No | PD |
| Patient D4 | CLL | 3/10;3/10 | 21 | 2 (7) | 0 | None | tDLI (69,90) | Yes (14) | PD |
| Patient D5 | NHL | 2/10;4/10 | 35 | 99 (36) | 99 (36) | IV | none | No | PF |
| Patient D6 | HD | 2/10;2/10 | 7 | 98 (126) | 98 (21) | II post DLI | tDLI (126) | No | CR |
NHL: non hodgkin lymphoma, HD, Hodgkin disease, AML: acute myeloid leukemia, CLL: chronic lymphocytic leukemia
HLA MM: HLA mismatch, GVH: graft versus host, HVG: host versus graft
p/t DLI: prophylactic/therapeutic donor lymphocyte infusion
PD: progressive disease, PR: partial remission, PF: progression free, CR: complete remission at the end of the study
all: chimerism on whole PBL
NA: not available
Chimerism data on Protocols A through C have been previously published [9] and are shown here to facilitate interpretation of subsequent data in this paper and to permit comparison to Protocol D.
The clinical details of and rationale for protocols A, B, and C are described elsewhere [9]. In all three protocols, cyclophosphamide, (50 mg/kg) was administered on Days −5, −4 and −3. The four patients treated with Protocol A received a MEDI-507 test dose of 0.1 mg/kg on Day −2 followed by therapeutic doses of 0.6 mg/kg on Days −1, 0 and +1. Protocol B included the test dose of MEDI-507 on Day −7 followed by doses of 0.6 mg/kg on each of Days −5 and −4. In both of these regimens, unmodified bone-marrow cells were administered. For Protocol C, the regimen was modified to include ex vivo T cell-depletion of G-CSF-mobilized peripheral blood stem cells (PBSC), using an IsolexR (Baxter Oncology, Deerfield, IL) CD34+ progenitor cell positive selection device. MEDI-507 dosing was the same as in Protocol B. The median number (range) of infused CD34+ and CD3 residual T cells was 10.6 (5.0-14.9×106)/kg and 8.9 (6.7-16 ×104)/kg respectively. To improve durable engraftment, Protocol D patients received the test dose of MEDI-507 on Day −8 followed by doses of 0.6 mg/kg on each of Days −7 and −6, cyclophosphamide (60mg/kg) on Days −7 and −6 and Fludarabine was given on Days −5 through −1 at 25mg/m2/d. The median number (range) of infused CD34+ and CD3 residual T cells was 8.4 (3.8-14.3×106)/kg and 4.25 (0.5-11.8×104)/kg respectively.
Flow Cytometry and cell sorting
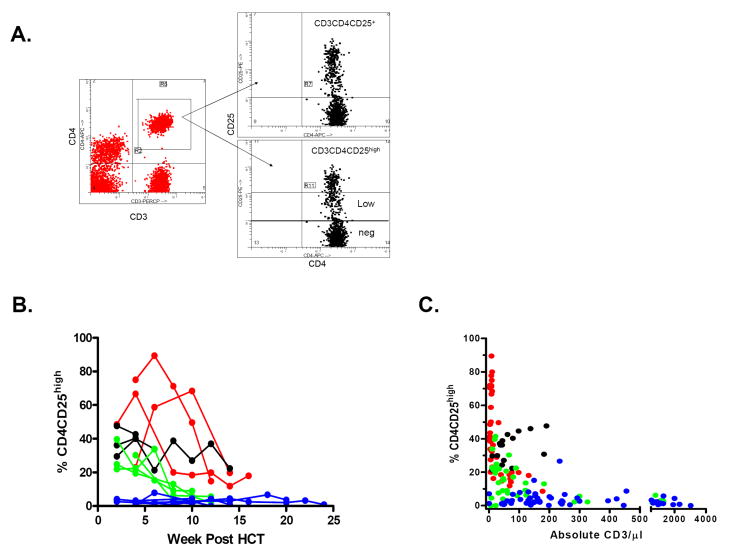
Multicolor flow cytometry was used to analyze white blood cells stained with labeled anti-HLA class I allele-specific monoclonal antibodies (One Lambda, Inc. Canoga Park, CA), and anti-CD3, CD4, CD8, CD19, CD25 and CD56, CD45RA, CD45RO, CD62L, CD25, and CD69 (Becton Dickinson, Mountain View, CA). For cell sorting of CD4+CD25high, dim or negative cells, frozen mononuclear cells from patient samples were thawed, treated with DNase, labeled with CD4-FITC and CD25-PE and analyzed/sorted by flow cytometry according to the gating as shown in Figure 3A.
Figure 3. CD25 expression on recovering CD4 T cells following non-myeloablative SCT.
A: Gating for analysis of CD3+CD4+ cell subpopulations in a representative patient with a high proportion of CD3+CD4+CD25high cells. Gates for the sorting of the CD3+CD4+CD25 high, low and negative (neg) populations are indicated.
B: Proportions of CD3+CD4+CD25high cells over time after HCT for individual patients (Red: Protocol A, Black: Protocol B, Green: Protocol C, Blue: Protocol D.)
C: Inverse relationship between CD25 expression on CD4 T cells and CD3 T cell recovery in recipients of MEDI-507-based non myeloablative haploidentical SCT protocol. (Red= Protocol A, Black=Protocol B, Green=Protocol C, Blue=Protocol D.) P<0.0001 and <0.006 for Protocols A and C, respectively. Patient B1 was excluded from analysis after the administration of anti-CD25 mAb Daclizumab (Zenapax) on Day 45 for the treatment of GVHD. Patient B4, who demonstrated an unusual T cell phenotype in which the majority of CD3+ T cells expressed CD45RO, CD45RA, and CD25 at all times, was also excluded from analysis. Patient D3 had CD4+CD25+ HTLV-1 lymphoma detectable in the peripheral blood, and therefore was excluded from this analysis.
Microsatellite analysis for chimerism
CD3 positive cells were isolated from ficoll-separated peripheral blood mononuclear cells (PBMC) with MACS beads (Miltenyi Biotech, Sunnyvale, CA) or with RosetteSep enrichment cocktail (Stem Cell Technologies, Inc., Vancouver, Canada) and DNA was extracted. PCR was performed using primers which flank the variable number of tandem repeats (VNTR) sequences or short terminal repeats (STR), as described [6].
Mixed lymphocyte culture (MLR) and cell mediated lysis (CML) assays
MLR and CML assays were performed on thawed PBMC and analyzed as described [16].
Limiting dilution assay (LDA) for cytotoxic T-lymphocyte precursor frequencies (CTLp) and IL-2-producing (helper) T cell frequencies (HTLf)
Graded numbers of responder PBMC were tested in 24 replicate wells each as previously described [16]. CTLp were measured based on the lysis of 51Cr labeled targets. For HTLf analysis, IL-2 production was measured as described [16]. For both CTLpf and HTLf assays, positive wells were defined as those producing cpm greater than 3 standard deviations above the mean of the 24 control wells containing stimulators alone.
Quantitative-PCR
Total RNA was extracted from 100 to 1000 FACS-sorted CD4+CD25 high, low or negative cells using the RNeasy MicroKit according to the manufacturer's protocol (QIAGEN). Total RNA from each sample was used as a template for the reverse transcription reaction. 20 μl of cDNA was synthesized using random hexamers, and SuperScript™ III RT (InVitrogen). All samples were reverse transcribed under the same conditions (25°C for 10 minutes, 50°C for 50 minutes) and from the same reverse transcription master mix in order to minimize differences in reverse transcription efficiency. The 25 μl QPCR reactions contained 2 μl of cDNA, 12.5 μl of 2x SYBR Green Master Mix (Stratagene), and 250 nmol of sense and antisense primer. Emitted fluorescence for each reaction was measured during the annealing/extension phase, and amplification plots were analyzed using MX4000 software, version 3.0 (Stratagene). A series of standards was prepared by performing 10-fold serial dilutions of full-length cDNAs in the range of 20 million copies to 2 copies per QPCR reaction. The calculated number of copies was divided by the number of copies of the housekeeping gene β2-microglobulin.
Statistical Analysis
Statistical analyses were performed with the non-parametric Wilcoxon-Rank Sum test or the non-parametric Spearman test. A value of p<0.05 was considered significant.
Results
Chimerism
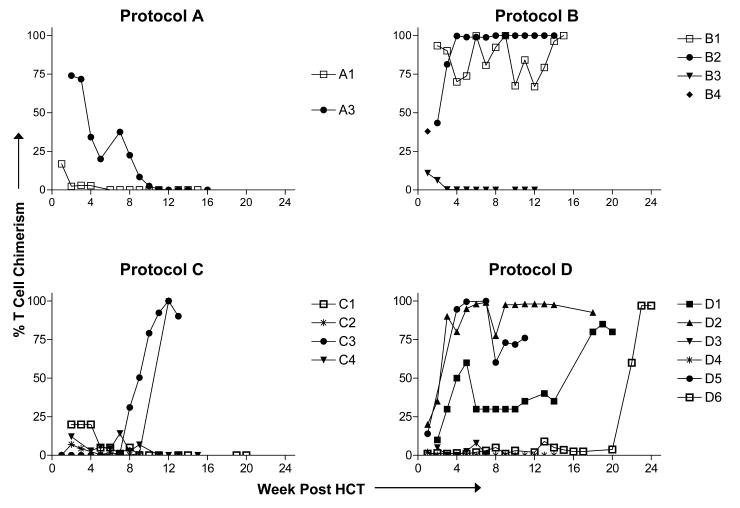
T cell and myeloid chimerism was assessed in PBMC samples collected approximately weekly through 100 days and at 6 months, 1 year and yearly thereafter when possible. Chimerism data are presented in Table I and Figure 1. In all patients except B3, maximum myeloid chimerism correlated with T cell chimerism. All four Protocol A patients achieved initial T chimerism, which became undetectable by 11 weeks and was not rescued by DLI. Two of three evaluable Protocol B patients (B1 and B2) developed full or near full donor T cell chimerism by 6-7 weeks without DLI, whereas Patient B3 had only transient chimerism. All four Protocol C patients showed low levels of initial T cell chimerism. Patient C1 gradually lost chimerism despite receiving three DLI. Patient C2 received DLI on Day 66 when her T cells were 1% donor-derived, and rapidly converted to 100% donor T cell chimerism by Day 90. Patient C3 received a large DLI (2.5×107 T cells/kg) on Day 51 and progressively increased T cell chimerism to >90% donor. Patient C4 showed initial T cell chimerism, which became undetectable by 11 weeks, despite administration of two DLI.
Figure 1. T cell chimerism after non-myeloablative haploidentical HCT.
In 16 of 18 patients we were able to distinguish donor from host T cells by the use of donor or host HLA allele-specific mAbs or by VNTR amplification of CD3+ cell fractions. T cell chimerism was determined using HLA allele-specific monoclonal antibodies in donor/recipient combinations for whom they were available (N=10) or VNTR of MACS-sorted CD3+ cells for the others. Data from individual patients is shown. Patients A2 and A4 are not represented because T cell separation was not performed. Chimerism data on Protocols A through C have been previously published [9] and are shown to facilitate interpretation of subsequent data in this paper and to permit comparison to Protocol D.
Protocol D differed from Protocol C mainly by the addition of fludarabine. Three of six Protocol D patients (Patients D1, D2, and D5) developed full or near full donor T cell chimerism without DLI, and two converted to full donor chimerism following DLI. Patient D4 showed <5% donor T cell chimerism at 1 week post-HCT, and despite receiving two DLI, failed to sustain measurable chimerism.
T Cell Recovery
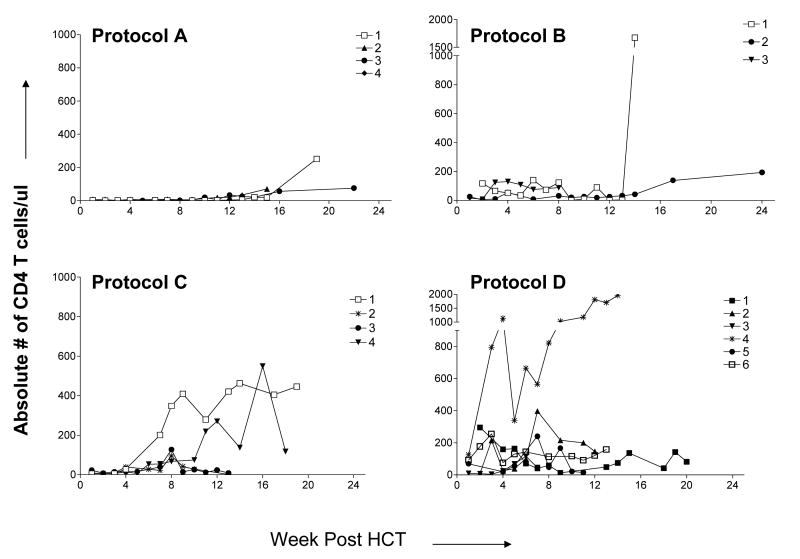
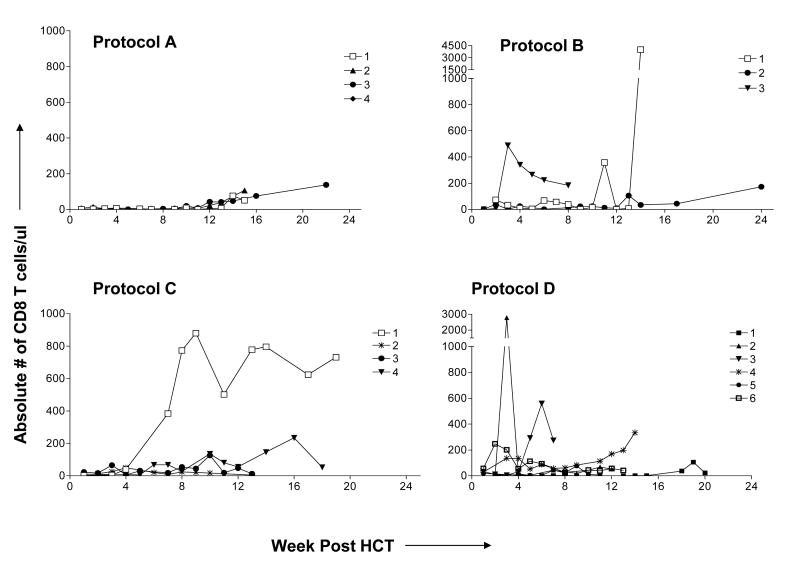
The day post-HCT when CD3+T cell numbers reached >100 cells/μl is indicated in Table I. In Protocol A, T cell recovery was slower compared to the three other groups (p<0.05 after 2 months). T cell subset recovery is shown in Figure 2A and B The recovery of both CD4 and CD8 subsets was extremely low in the first 100 days post-HCT in recipients of Protocol A. In Protocol B, recovery of both subsets was variable between patients. T cell recovery among recipients of Protocol C was most rapid in the 2 patients who lost their grafts (Patients C1 and C4), including both CD4 and CD8 cells. Despite the addition of fludarabine, Protocol D T cell recovery was significantly greater than that in Protocol C (and A) at 4 weeks (p<0.03 and <0.01 respectively).
Figure 2a. CD4 T cell recovery post-HCT.
The absolute number of CD4 T cells/μl was calculated by multiplying the absolute lymphocyte count by the percentage of CD3+CD4+ cells among lymphocytes. Patient B4, who died early from multiple organ failure, is not reported.
Figure 2b. CD8 T cell recovery post-HCT.
The absolute number of CD8 T cells/μl was calculated by multiplying the absolute lymphocyte count by the percentage of CD3+CD8+ cells among lymphocytes. Patient B4, who died early from multiple organ failure, is not reported.
CD4 cells appearing in the first 100 days post-HCT were CD45RA−/CD45RO+ “memory” cells. Likewise, only a small proportion of recovering CD3+CD8+ cells expressed the CD45RA+ CD45RO-CD62L+ “naive” phenotype in all groups (data not shown).
High proportion of CD25+ cells among initially recovering CD4 T cells
Although T cell counts were initially very low in most patients, very large numbers (105) of total events were analyzed whenever possible, so that meaningful phenotypic data on CD25 expression could be obtained. Early after transplantation, CD4+CD25+ T cells represented high percentages of CD4+ T cells. The intensity of CD25 staining was heterogeneous, with a distinct population of CD4+CD25high cells (e.g. Figure 3A).
At 2 weeks post-HCT, the median percentage of CD4 T cells expressing CD25 in all groups was 80.9% (67.9-84.4%). By 12 weeks, this percentage decreased to a median of 30.2% (23.3-59.3%). There was no difference in CD25 expression between patients on Protocols A, B and C, or between patients that lost or maintained chimerism, or in association with GVHD. As shown in Figure 3B, high proportions of CD3+CD4+ CD25high cells were initially present in most recipients of Protocols A, B, and C and correlated inversely with absolute T cell counts in Protocols A and C (Figure 3C) (p<0.001). The increased percentage of CD25high CD4 cells early vs late post-transplant was due to relative enrichment, not to an increase in absolute numbers of CD25high cells at early time points (data not shown). However, Protocol D patients showed lower percentages of CD4+CD25high T cells than those in Protocols A, B, and C in the first three months (Figure 3B) (p<0.03). CD3+CD8+ cells generally included much lower proportions of CD25+ cells, with only sporadic increases in a few patients (data not shown).
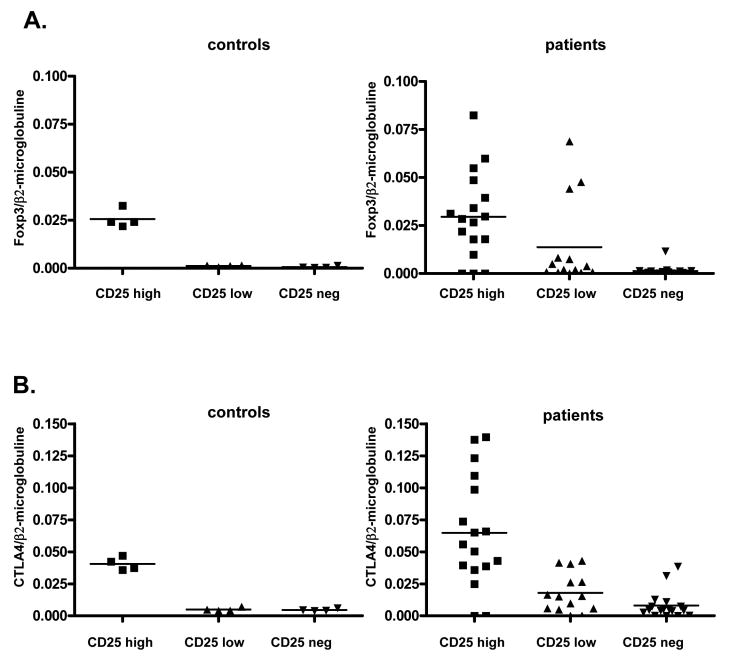
CD4+CD25high cells express Foxp3 and CTLA4
Since CD25 is expressed both by activated CD4+ T cells and by regulatory CD4 T cells, we performed further studies to characterize CD425+CD4+ populations. The severe T cell lymphopenia following conditioning precluded performance of functional assays. Therefore we assessed regulatory populations by quantitative (q)RT-PCR assessment of mRNA for Foxp3 [17-22] and CTLA4, which are considered to be the most reliable marker of Tregs [18-22]. We analyzed 17 samples from 7 patients in various groups during the first 100 days post-transplant. One hundred to one thousand thawed cells were sorted and analyzed and compared to similarly-processed samples from healthy donors.
CD25high CD4+ cells from controls expressed higher levels of Foxp3 and CTLA mRNA than CD25low or CD25− CD4 cells, demonstrating Treg enrichment among CD25high cells. As shown in Figure 4A and 4B, CD4+CD25high cells from patients had more heterogeneous FOXP3 and CTLA-4 mRNA levels, but these were generally higher or similar to those in normal controls (Figure 4A and 4B).
Figure 4. Foxp3 and CTLA-4 expression in CD4+ T cells.
A. Foxp3 expression is shown according to the expression of CD25 in the CD4+ T cell population: Patient CD25high, CD25low and CD25− populations compared to normal donors
B. CTLA-4 expression is shown according to the expression of CD25 in the CD4+ T cell population: Patient CD25 high, low and negative CD4 cell populations compared to a group of normal donors.
Samples tested: patient A1 at Days 69, 77, and 92, Patient A2 at Days 56 and 98, Patient A3 at Days 33, 46 and 55, Patient B2 at Days 49, 71 and 99, Patient C4 at Days 81 and 100, Patient D5 at Days 50 and 70, Patient D6 at Days 22 and 70 (the same samples are tested for cytokine expression in Figure 5).
Two of three CD4+CD25high samples that did not express Foxp3 were from Patient A1 shortly following DLI and also failed to express CTLA-4. Two weeks later, at Day 92, the CD4+CD25high cells in this patient, which were all host-derived, expressed both Foxp3 and CTLA-4. A Foxp3-negative sample from Patient D5 was obtained 13 days after infusion of pre-transplant recipient lymphocytes to treat refractory GHVD.
The infusion resulted in a decline in T cell chimerism from 100% to 60%. In a sample taken 20 days before lymphocyte infusion, sorted CD4+CD25high T cells expressed both Foxp3 and CTLA-4.
Foxp3 and CTLA-4 expression were also more heterogeneous in CD4+CD25low cells of patients than normal controls (Figure 4A,B). Three CD4+CD25low samples from 3 Group A patients expressed Foxp3 (Figure 4A) and 5 CD4+CD25low samples from Patients A1, A2, B2, and D6 expressed increased CTLA-4 mRNA compared to controls (Figure 4B). Among CD4+CD25− samples, only one patient sample expressed a low level of Foxp3, and 2 samples from two patients expressed lows of level CTLA-4 (Figure 4A,B).
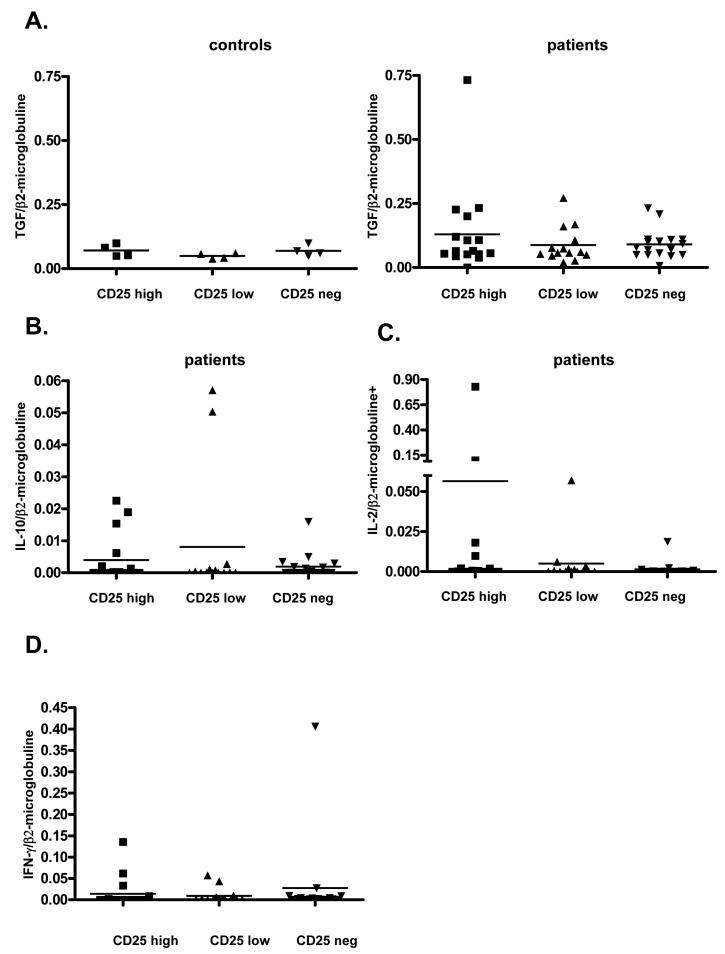
Cytokine Expression Profile of CD4 Subpopulations
We used qRT-PCR to compare the expression of cytokines associated with Tregs (TGF-β and IL-10) [23-26] and T cell activation (IL-2, IFN-γ) [27-30] in the above CD4 subpopulations. All sorted CD4+ T cell populations expressed a basal level of TGF-β mRNA, in both controls and patient samples, with no statistical difference. CD4+CD25high cells from one patient from Group A expressed a high level of TGF-β mRNA (Figure 5A). We did not detect IL-10 mRNA in the control group (data not shown). Seven patient samples expressed low levels of IL-10 among all three CD4+ populations (Figure 5B).
Figure 5. Cytokine expression in CD4+ T cells.
A. TGF-β expression is shown according to the expression of CD25 in the CD4+ T cell population: Patient CD25high, low and negative CD4 cell populations compared to a group of normal donors.
B. IL-10 expression is shown according to the expression of CD25 in the CD4+ T cell population.
C. IL-2 expression is shown according to the expression of CD25 in the CD4+ T cell population: Y axis has a broken scale.
D. IFN-γ expression is shown according to the expression of CD25 in the CD4+ T cell population: Y axis has a broken scale.
Messenger RNA for IL-2 was not expressed in control samples (data not shown) but was expressed in some samples from Protocol A recipients. The only CD4+CD25high population with very high IL-2 expression was a Patient A1 sample that did not express Foxp3 or CTLA4 (Day 69). Thus, these CD4+CD25high cells appeared to be activated CD4+ T cells (Figure 5C) of recipient origin (since donor chimerism was not detected) in this patient who had just received a DLI before the sample was obtained.
IFN-γ mRNA was undetectable in control samples (data not shown). Three CD4+CD25high patient samples expressed IFN-γ mRNA, including the above IL-2-positive post-DLI sample from Patient A1 (Day 69), Patient A3 at Day 33, when a capillary leak syndrome was present (the sample was also FoxP3+) and Patient D5 at Day 70, 13 days following infusion of pre-transplant recipient lymphocytes (see above). The IFN-γ+ samples from Patients A1 and D5 did not express Foxp3. One sample also expressed a high level of IFN-γ in the CD4+CD25− population (Patient A3 at Day 69) (Figure 5D).
Alloreactivity of T cells recovering after non-myeloablative haploidentical HCT with Medi-507
In vitro alloresponses to donor, host and third party were evaluated when sufficient cells were available using MLR and CML for Protocols A, B and C or HTLp/CTLp for protocol D. Patient A2, who lost her graft on Day 21, had a weak anti-third party MLR response (stimulation index (SI = 3.0) and a measurable anti-donor MLR response (SI = 5.5) on Day 55. Assays performed on later samples were unresponsive to both donor and third party. Patient A3, who also lost her graft, had CML assessments at Days 33, 46, 82 and 122, and showed no anti-donor response. Subsequent assays at 6 and 11 months showed weak anti-donor CTL responses (<15% specific lysis). CTL activity against third party was not assessed at these times. An anti-donor (SI =8.1) but not a third party MLR response was measurable at Day 122. Patient B3, who lost his graft by 3 weeks, had no measurable anti-donor or third party MLRs at Days 35 and 42 (data not shown). Patient C1, who rejected his donor graft by Day 77, did not show anti-donor or third party responses on Day 70 or 100, but later (Day 134) recovered a strong anti-third party (SI=102) and anti-donor MLR response (SI=40), with a weak but measurable anti-donor (maximum PSL 20%) and unmeasurable third party CML response. Patient C3, who had durable chimerism, had negative anti-donor and anti-third party MLR responses at Days 51 and 71 (data not shown). Thus, in vitro responses were globally suppressed early post-transplant. These assays did not reveal anti-donor responses prior to graft loss, but anti-donor MLR and/or CML responsiveness was detectable in three of four patients following graft loss.
LDA were performed on 4 patients from Group D. In Patient D1, who lacked an anti-donor HTLp response prior to HCT, HTLp reactivity was detected against both host and third party following spontaneous conversion toward full donor chimerism on Day 105 post-transplant (Table II).
Table 2. Results of limiting dilution assays (CTLp and HTLp) for 4 patients from protocol D.
| CTLp | HTLp | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Donor | PreTx Host | 3rd Party | Donor | PreTx Host | 3rd Party | |
| D1 | Donor | ND | 1:59,772 | ND | ND | 1:49,493 | ND |
| PreBMT | ND | 1:210,619 | ND | ND | Neg | 1:208,383 | |
| Day 70 | ND | ND | ND | ND | Neg | Neg | |
| Day 105 | ND | ND | ND | ND | 1:12,205 | 1:39,821 | |
| Day 287 | ND | 1:269,169 | 1:27,974 | ND | 1:157,165 | Neg | |
| D4 | Donor | Neg | Neg | ND | Neg | 1:230,205 | ND |
| PreBMT | Neg | Neg | ND | 1:73,639 | Neg | ND | |
| Day 10 | Neg | Neg | ND | 1:8,084 | 1:377,304 | ND | |
| Day 42 | Neg | Neg | Neg | 1:20,991 | Neg | Neg | |
| Day 70 | Neg | Neg | Neg | 1:380,874 | Neg | Neg | |
| D5 | Donor | 1:71621 | 1:17327 | ND | Neg | 1:56216 | ND |
| PreTx | 1:98396 | Neg | ND | 1:17637 | Neg | ND | |
| Day 50 | Neg | Neg | Neg | Neg | Neg | 1:10678 | |
| Day 70 | Neg | Neg | Neg | 1:14788 | Neg | 1:7424 | |
| D6 | Donor | Neg | 1:23,115 | ND | Neg | 1:32,999 | ND |
| PreTx | 1:6,248 | Neg | ND | 1:15,887 | Neg | ND | |
| Day 21 | Neg | 1:107,727 | Neg | 1:111,167 | 1:95,961 | 1:17,040 | |
| Day 98 | Neg | 1:148,374 | 1:17,562 | Neg | Neg | 1:5,539 | |
Neg = no measurable response as calculated via the Poisson distribution
ND = not done
In Patient D4, chimerism became undetectable by Day 14 post-HCT, but no CTLp response against the donor, host, or third party was detected on Days 10, 42 or 70 (Table II). However, a strong and elevated (compared to pre-transplant) anti-donor HTLp response was detectable by Day 10 (Table II). This patient's haploidentical sibling donor was HLA A and B mismatched (see Table I), HLA-DR and HLA-DQ identical at the molecular level, but HLA-DP mismatched, suggesting that rejection might have been directed at the donor DP allele.
Patient D5, who converted spontaneously to full donor chimerism 2-4 weeks post-transplant, was evaluated pre-transplant and on Days 50 and 70 post-HCT. Although donor anti-host responses were detectable and the patient showed anti-donor reactivity pre-transplant, neither response was apparent in the patient on Day 50, when a third party response had recovered (Table II). At this time the patient developed severe GVHD (grade III/IV) which was unresponsive to multiple therapies. In an effort to reverse GVHD, the patient received an infusion of autologous PBMC on Day 57. At Day 70, a strong anti-donor HTLp response became detectable (Table II) in association with a decline in T cell chimerism from 100% to 60% (Figure 1, Patient D5).
Patient D6 had a period of sustained mixed chimerism before DLI was administered on Day 126, providing an opportunity to examine tolerance in a stable mixed chimera. At both 21 and 98 days, granulocytes were 98% donor, but less than 1% and only 5-10% of T cells were of donor origin at Days 21 and 98, respectively. As shown in Table II, a high frequency of anti-donor HTLp was present in the recipient pre-transplant, declined markedly by Day 21, and became undetectable by Day 98. Strong anti-third party responses were detected at all time points, even by 21 days post-transplant. While the donor had a strong anti-recipient HTLp frequency, anti-host responses were very low in the patient by Day 21 and essentially undetectable by Day 98 post-transplant. Thus, this patient demonstrated donor- and host-specific unresponsiveness during the period of stable mixed chimerism.
Discussion
Achieving reliable engraftment, anti-tumor effects and immune reconstitution without GVHD across haplotype barriers remains a major challenge in clinical HCT [6,31-37]. We aim to use in vivo and/or ex vivo T cell-depleted donor HCT to establish mixed chimerism without a GVHR, creating a platform for adoptive cellular immunotherapy using DLI to achieve GVL without GVHD [2-4]. We have progressively developed four variations of a regimen that includes cyclosphosphamide, thymic irradiation and MEDI-507, followed by a short course of cyclosporine. The first protocols (A and B) involved administration of unmodified donor marrow, relying on in vivo T cell depletion by Medi-507. CD34-selected PBSC were instead utilized in Protocols C and D, and fludarabine was added to Protocol D. While optimization of this regimen is still in progress, results reported previously from these protocols [9] provide proof of principle that: 1) sustained mixed chimerism can be achieved across HLA haplotype barriers with non-myeloablative conditioning; and 2) sizeable DLI can be given to patients with haplotype mismatched mixed chimerism, without inducing severe GVHD, converting them toward full donor T cell chimerism.
Protocol D involved the addition of fludarabine in an effort to more reliably achieve sustained engraftment. Five of six patients achieved sustained engraftment, compared to 2 of 4 recipients of the same regimen without fludarabine, suggesting that fludarabine may enhance the engraftment of haploidentical CD34 cells. Despite the use of similar CD34-selected PBSC inocula in Protocols C and D, only patients in Protocol D (2 of 6) converted spontaneously to full donor chimerism and developed GVHD without DLI, raising the possibility that fludarabine might increase donor engraftment and susceptibility to GVHD from small numbers of residual T cells in the donor inoculum, possibly by depleting recipient regulatory T cells, which may attenuate the GVH response, and with it, donor engraftment and GVHD. Only fludarabine-treated patients failed to show marked enrichment for these cells early post-transplant, as is discussed below. However, the small cohort numbers preclude the formation of conclusions on clinical outcomes at this time.
Our data demonstrate enrichment for regulatory T cells [38,39] early post-transplant in Protocols A, B and C, but not Protocol D. These CD25high CD4+ T cells expressed high levels of FoxP3 and CTLA4 and in most cases did not express proinflammatory cytokines (IL-2 or IFN-γ). Foxp3 and CTLA-4 were expressed at similar or lower levels in CD4+CD25high populations in normal controls, suggesting a similar or greater frequency of regulatory cells among CD25high CD4 cells of the patients. While CTLA-4 is also expressed on activated non-regulatory T cells in addition to Treg [40,41], FoxP3 is mainly associated with Treg activity [17-22]. Because the vast majority of patient CD4+ T cells had a “memory” phenotype, the predominant CD4+CD25high cells are likely to be residual T cells escaping depletion. The reduced number of these cells in recipients of Protocol D compared to Protocol C suggests that fludarabine impairs recipient Treg survival. Consistently, chronic lymphocytic leukemia patients receiving fludarabine treatment showed decreased numbers of CD4+CD25high Treg due to increased apoptosis [42]. This observation may help to explain the relatively high incidence of acute GVHD that is observed in association with fludarabine-based non-myeloablative conditioning regimens [43]. However, our data do not allow us to firmly state that only recipient Treg survival or recovery was affected by fludarabine, since we did not assess chimerism among T cells with the Treg phenotype, and mixed T cell chimerism was present in many of the samples showing Treg predominance.
The early enrichment for Treg in recipients of Protocols A, B and C, correlated strongly with low total T cell counts. In fact, no increase in the absolute concentration of Treg above normal levels was seen in patients (data not shown), suggesting that their enrichment reflects relative resistance of Treg to depletion by Medi-507. Recent in vitro studies showed that ATG can induce immune unresponsiveness due to the expansion/activation of CD4+ T reg [44]. We have recently described marked enrichment of CD25high CD4+ T cells in patients receiving ATG-based non-myeloablative allogeneic bone marrow transplantation with a kidney graft from the same donor [45]. While studies are in progress to determine whether or not these are indeed Treg, it is tempting to speculate that they may have contributed to the renal allograft tolerance detected in patients who lost donor chimerism.
In mice, CD4+CD25+ Treg can prevent GVHD [46-51] while maintaining GVL [52] and can promote engraftment and tolerance [53]. However, clinical data are somewhat conflicting, showing decreased numbers in association with acute GVHD [54] and increased or decreased Treg in association with acute or chronic GVHD in various studies [53-55]. These studies focused mainly on HLA-identical related or unrelated HCT following myeloablative conditioning. While a recent study showed early Treg recovery after autologous transplantation for autoimmune disease [56], to our knowledge, ours is the first study to demonstrate predominant recovery of Treg following allogeneic HCT.
While TGF-β and IL-10 have been frequently implicated in Treg function [23-26], all CD4 T cell subsets in our patients and controls expressed a low level of TGF-β mRNA. IL-10 was detected in a few samples, but not specifically in the CD4+CD25high population. The absence of Foxp3 and presence of IFN-γ (with or without IL-2) expression in three CD4+CD25high samples from 2 different patients following donor or recipient lymphocyte infusion suggests that these were activated T cells. Thus, in the context of in vivo alloresponses, CD4CD25high cell populations may include activated T cells.
Haploidentical pairs normally have pre-existing bulk T cell responses to each other. However, patients in Protocols A, B and C who lost chimerism had measurable anti-donor CTL and/or MLR responses only following and not prior to the loss of chimerism. The weak anti-third party responses early post-transplant period are consistent with the low T cell counts and predominance of Treg in this period. These data do not point to anti-donor immune responses as the cause of loss of chimerism, but do not rule it out. Of note, patients with transient chimerism following combined haploidentical renal and bone-marrow transplantation under treatment similar to Protocol A have demonstrated renal allograft tolerance and CD25+CD4+ T cell-mediated suppression of anti-donor responses in vitro (T. Kawai et al, manuscript in preparation).
In contrast to the above group, recipient D4 rapidly lost chimerism without Treg enrichment in PBMC and showed a strong and increased (compared to pre-transplant) anti-donor Th response, without anti-donor CTL, by 10 days post-HCT. This response was likely directed toward a donor DP allele, since the donor and recipient were matched at DR and DQ. DP mismatching has been variably associated with GVHD and rejection in unrelated donor transplantation [57-59], but in association with CTL responses [60,61]. Our data are consistent with our previous study suggesting that rejection may be mediated by Th without CTL [16] and suggest DP polymorphisms as a possible target.
In another patient receiving Protocol D, infusion of recipient lymphocytes to treat refractory GVHD was associated with improvement in GVHD, a decline in donor T cell chimerism, and a strong anti-donor Th response. This patient's CD4+CD25high population expressed Foxp3 prior to the infusion, but became Foxp3− and IFN-γ+ when strong anti-donor HTLp appeared following recipient PBMC infusion, suggesting T cell activation.
HTLp analysis on a stable mixed chimera revealed donor- and recipient-specific unresponsiveness, with rapid recovery of anti-third party responses. This, to our knowledge, is the first reported analysis of alloresponses in a human stable mixed allogeneic chimera prepared across HLA barriers and indicates that, as in the mouse model [62,63], systemic donor- and host-specific tolerance can be achieved by the induction of mixed allogeneic chimerism. This result provides proof of principle that mixed hematopoietic chimerism could be used to achieve systemic donor- and host-specific tolerance across HLA barriers for the induction of organ allograft tolerance and the treatment of non-malignant hematologic disorders.
Acknowledgments
We thank Dr. Beow Yeap for statistical advice, Drs. Jerry Ritz and Emmanuel Zorn for helpful review of the manuscript, and Ms. Kelly Walsh for expert assistance in preparing the manuscript.
This work was supported by NIH grants RO1 HL63474, RO1 CA79986 and RO1 CA79989.
Footnotes
The authors have nothing to declare regarding the declaration of commercial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelot MR, Pearson DA, Swenson K, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5:133–143. doi: 10.1053/bbmt.1999.v5.pm10392959. [DOI] [PubMed] [Google Scholar]
- 3.Mapara MY, Pelot M, Zhao G, Swenson K, Pearson D, Sykes M. Induction of stable long-term mixed hematopoietic chimerism following nonmyeloablative conditioning with T cell-depleting antibodies, cyclophosphamide, and thymic irradiation leads to donor-specific in vitro and in vivo tolerance. Biol Blood Marrow Transplant. 2001;7:646–655. doi: 10.1053/bbmt.2001.v7.pm11787527. [DOI] [PubMed] [Google Scholar]
- 4.Mapara MY, Kim Y-M, Wang S-P, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903–1909. doi: 10.1182/blood-2002-01-0023. [DOI] [PubMed] [Google Scholar]
- 5.Sykes M, Preffer F, McAffee S, et al. Mixed lymphohematopoietic chimerism and graft-vs-lymphoma effects are achievable in adult humans following non-myeloablative therapy and HLA-mismatched donor bone marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer TR, MCafee S, Sackstein R, et al. The intentional induction of mixed chimerism and achievement of anti-tumor responses following non-myeloablative conditioning therapy and HLA-matched and mismatched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6:309–320. doi: 10.1016/s1083-8791(00)70056-5. [DOI] [PubMed] [Google Scholar]
- 7.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. New Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 8.Martelli MF, Aversa F, Bachar-Lustig E, et al. Transplants across human leukocyte antigen barriers. Semin Hematol. 2002;39:48–56. doi: 10.1053/shem.2002.29255. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer TR, MCafee S, Dey BR, et al. Non-myeloablative haploidentical stem cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–1751. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 10.Henslee-Downey PJ. Mismatched bone marrow transplantation. Curr Opin Oncol. 1995;2:115–121. doi: 10.1097/00001622-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Butturini A, Gale RP. The role of T-cells in preventing relapse in chronic myelogenous leukemia. Bone Marrow Transplant. 1987;2:351–354. [PubMed] [Google Scholar]
- 12.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 13.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T cell-depleted stem cells from related donors with one fully mismatched haplotype. New Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 14.Chakraverty R, Cote D, Buchli J et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006 doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mapara MY, Kim YM, Marx J, Sykes M. DLI-mediated GVL effects in mixed chimeras established with a non-myeloablative conditioning regimen: extinction of GVL effects coincides with loss of alloreactive cells following conversion to full donor chimerism. Transplantation. 2003;76:297–305. doi: 10.1097/01.TP.0000072014.83469.2D. [DOI] [PubMed] [Google Scholar]
- 16.Kraus AB, Shaffer J, Toh HC, et al. Early host CD8 T-cell recovery and sensitized anti-donor IL-2-producing and cytolytic T-cell responses associated with marrow graft rejection following nonmyeloablative bone marrow transplantation. Exp Hematol. 2003;31:609–621. doi: 10.1016/s0301-472x(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 19.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4(+)CD25(+) T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 21.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 22.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 24.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-{beta}1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1003. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 28.Pietryga DW, Blazar BR, Soderling CCB, Vallera DA. The effect of T subset depletion on the incidence of lethal graft-versus-host disease in a murine major-histocompatibility-mismatched transplantation system. Transplantation. 1987;43:442–445. doi: 10.1097/00007890-198703000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad M, Lecron JC, Descombe JJ, Tanzer J, Goube de Laforest P. Endogenous production of prothymocyte differentiating activity by phytohemagglutinin-stimulated T-cell-depleted human marrow. Cell Immuol. 1986;103:299–310. doi: 10.1016/0008-8749(86)90091-2. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan KE, Cutilli J, Piliero LM, et al. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2000;7:920–924. doi: 10.1128/cdli.7.6.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzieri DA, Long GD, Vredenburgh JJ, et al. Chimerism mediated immunotherapy using Campath T-cell depleted peripheral blood progenitor cells (PBSC) with nonmyeloablative therapy provides reliable, durable allogeneic engraftment. Blood. 2000;96:521a. [Google Scholar]
- 32.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents GvHD following nonmyeloablative stem-cell transplantation. Cytotherapy. 2001;3:197–201. doi: 10.1080/146532401753174025. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA- mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 34.Shimazaki C, Fuchida S, Ochiai N, et al. Non-T-cell-depleted HLA-haploidentical stem cell transplantation after reduced-intensity conditioning in advanced haematological malignancies based on feto-maternal microchimerism. Br J Haematol. 2004;127:474–475. doi: 10.1111/j.1365-2141.2004.05228.x. [DOI] [PubMed] [Google Scholar]
- 35.Shimazaki C, Ochiai N, Uchida R, et al. Non-T-cell-depleted HLA haploidentical stem cell transplantation in advanced hematologic malignancies based on the feto-maternal michrochimerism. Blood. 2003;101:3334–3336. doi: 10.1182/blood-2002-09-2883. [DOI] [PubMed] [Google Scholar]
- 36.Tamaki H, Ikegame K, Kawakami M, et al. Successful engraftment of HLA-haploidentical related transplants using nonmyeloablative conditioning with fludarabine, busulfan and anti-T-lymphocyte globulin. Leukemia. 2003;17:2052–2054. doi: 10.1038/sj.leu.2403092. [DOI] [PubMed] [Google Scholar]
- 37.Ichinohe T, Uchiyama T, Shimazaki C, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821–3828. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
- 38.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 40.Maszyna F, Hoff H, Kunkel D, Radbruch A, Brunner-Weinzierl MC. Diversity of clonal T cell proliferation is mediated by differential expression of CD152 (CTLA-4) on the cell surface of activated individual T lymphocytes. J Immunol. 2003;171:3459–3466. doi: 10.4049/jimmunol.171.7.3459. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Y, Funda DP, Every AL, et al. TCR-mediated activation promotes GITR upregulation in T cells and resistance to glucocorticoid-induced death. Int Immunol. 2004;16:1315–1321. doi: 10.1093/intimm/dxh134. [DOI] [PubMed] [Google Scholar]
- 42.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+ CD25high regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 43.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 44.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 45.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 46.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft- versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 48.Blazar BR, Lees CJ, Martin PJ, et al. Host T cells resist graft-versus-host disease mediated by donor leukocyte infusions. J Immunol. 2000;165:4901–4909. doi: 10.4049/jimmunol.165.9.4901. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez M, Quezada SA, Blazar BR, Panoskaltsis-Mortari A, Rudensky AY, Noelle RJ. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning. J Immunol. 2002;169:5581–5589. doi: 10.4049/jimmunol.169.10.5581. [DOI] [PubMed] [Google Scholar]
- 50.Johnson BD, Konkol MC, Truitt RL. CD25+ immunoregulatory T-cells of donor origin suppress alloreactivity after BMT. Biol Blood Marrow Transplant. 2002;8:525–535. doi: 10.1053/bbmt.2002.v8.pm12434947. [DOI] [PubMed] [Google Scholar]
- 51.Xia G, Truitt RL, Johnson BD. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol Blood Marrow Transplant. 2006;12:397–407. doi: 10.1016/j.bbmt.2005.11.519. [DOI] [PubMed] [Google Scholar]
- 52.Edinger M, Hoffmann P, Ermann J, et al. CD4(+)CD25(+) regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 53.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 54.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 55.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de K I, Vastert B, Klein M, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 57.Gallardo D, Brunet S, Torres A, et al. HLA-DPB1 mismatch in HLA-A-B-DRB1 identical sibling donor stem cell transplantation and acute graft-versus-host disease. Transplantation. 2004;77:1107–1110. doi: 10.1097/01.tp.0000122225.10296.10. [DOI] [PubMed] [Google Scholar]
- 58.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 59.Petersdorf EW, Smith AG, Mickelson EM, et al. The role of HLA-DPB1 disparity in the development of acute graft-versus-host disease following unrelated donor marrow transplantation. Blood. 1993;81:1923–1932. [PubMed] [Google Scholar]
- 60.Zino E, Frumento G, Marktel S, et al. A T-cell epitope encoded by a subset of HLA-DPB1 alleles determines nonpermissive mismatches for hematologic stem cell transplantation. Blood. 2004;103:1417–1424. doi: 10.1182/blood-2003-04-1279. [DOI] [PubMed] [Google Scholar]
- 61.Fleischhauer K, Locatelli F, Zecca M, et al. Graft rejection after unrelated donor hematopoietic stem cell transplantation for thalassemia is associated with nonpermissive HLA-DPB1 disparity in host-versus-graft direction. Blood. 2006;107:2984–2992. doi: 10.1182/blood-2005-08-3374. [DOI] [PubMed] [Google Scholar]
- 62.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 63.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]