Abstract
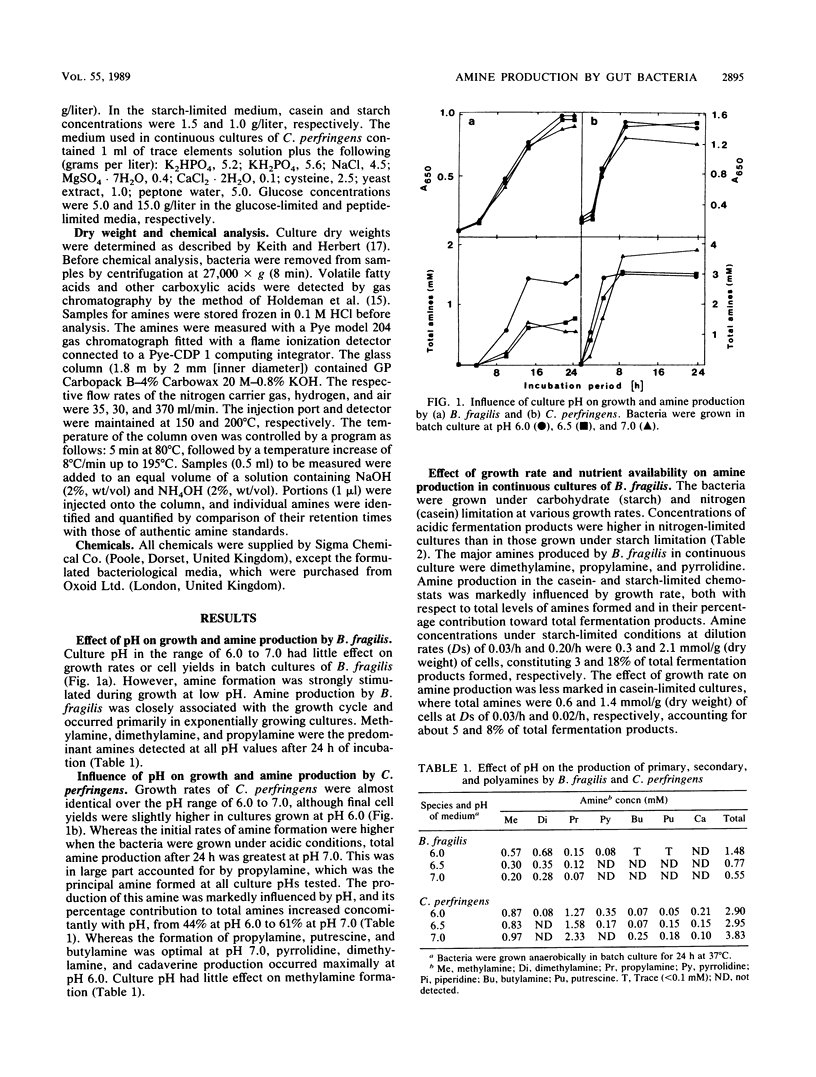
Dimethylamine, methylamine, propylamine, and pyrrolidine were the major amines formed by Bacteroides fragilis NCDO 2217 during the active phase of growth in batch culture. Production of these metabolites was strongly pH dependent and was optimal under acidic conditions (pH 6.0). Low pH also favored the formation of pyrrolidine, cadaverine, and dimethylamine by Clostridium perfringens C523, but the reverse was the case with putrescine, butylamine, and propylamine, where production was maximal at neutral pH. B. fragilis was grown in continuous culture under either starch or casein limitation. Amine formation was influenced by carbohydrate availability and was greatest when the bacteria were grown at high growth rates (dilution rate, 0.20/h) under starch limitation, where they constituted about 18% of the total fermentation products measured. Amine production was optimal and increased concomitantly with growth rate when C. perfringens was grown in glucose-limited continuous culture. Under conditions of high growth rate and glucose limitation, amines accounted for approximately 27% of the fermentation products measured. When glucose in the feed medium was increased from 5 to 15 g/liter, amine production was repressed, and under these nutritional conditions the growth rate had little effect on the process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison C., McFarlan C., MacFarlane G. T. Studies on mixed populations of human intestinal bacteria grown in single-stage and multistage continuous culture systems. Appl Environ Microbiol. 1989 Mar;55(3):672–678. doi: 10.1128/aem.55.3.672-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Calmels S., Ohshima H., Vincent P., Gounot A. M., Bartsch H. Screening of microorganisms for nitrosation catalysis at pH 7 and kinetic studies on nitrosamine formation from secondary amines by E. coli strains. Carcinogenesis. 1985 Jun;6(6):911–915. doi: 10.1093/carcin/6.6.911. [DOI] [PubMed] [Google Scholar]
- Chacko A., Cummings J. H. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988 Jun;29(6):809–815. doi: 10.1136/gut.29.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeQuattro V. L., Sjoerdsma A. Origin of urinary tyramine and tryptamine. Clin Chim Acta. 1967 May;16(2):227–233. doi: 10.1016/0009-8981(67)90185-4. [DOI] [PubMed] [Google Scholar]
- Fine D. H., Ross R., Rounbehler D. P., Silvergleid A., Song L. Formation in vivo of volatile N-nitrosamines in man after ingestion of cooked bacon and spinach. Nature. 1977 Feb 24;265(5596):753–755. doi: 10.1038/265753a0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Lockhart B. M. Rapid methods for determining decarboxylase activity: arginine decarboxylase. Appl Microbiol. 1971 Sep;22(3):350–357. doi: 10.1128/am.22.3.350-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A. The production of secondary amines by the human gut bacteria and its possible relevance to carcinogenesis. Med Lab Sci. 1977 Apr;34(2):131–143. [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Englyst H. N. Starch utilization by the human large intestinal microflora. J Appl Bacteriol. 1986 Mar;60(3):195–201. doi: 10.1111/j.1365-2672.1986.tb01073.x. [DOI] [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Boeker E. A. Biosynthetic and biodegradative ornithine and arginine decarboxylases from Escherichia coli. Methods Enzymol. 1983;94:125–134. doi: 10.1016/s0076-6879(83)94020-x. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Murray K. E., Adams R. F., Earl J. W., Shaw K. J. Studies of the free faecal amines of infants with gastroenteritis and of healthy infants. Gut. 1986 Oct;27(10):1173–1180. doi: 10.1136/gut.27.10.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHEAR E. A., RUEBNER B. The in vitro production of ammonium and amines by intestinal bacteria in relation to nitrogen toxicity as a factor in hepatic coma. Br J Exp Pathol. 1956 Jun;37(3):253–262. [PMC free article] [PubMed] [Google Scholar]
- Saul R. L., Kabir S. H., Cohen Z., Bruce W. R., Archer M. C. Reevaluation of nitrate and nitrite levels in the human intestine. Cancer Res. 1981 Jun;41(6):2280–2283. [PubMed] [Google Scholar]
- Shephard S. E., Schlatter C., Lutz W. K. Model risk analysis of nitrosatable compounds in the diet as precursors of potential endogenous carcinogens. IARC Sci Publ. 1987;(84):328–332. [PubMed] [Google Scholar]
- Suzuki K., Mitsuoka T. N-nitrosamine formation by intestinal bacteria. IARC Sci Publ. 1984;(57):275–281. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker L., Brooks J. B. In vitro production of N-nitrosodimethylamine and other amines by proteus species. Infect Immun. 1974 Apr;9(4):648–653. doi: 10.1128/iai.9.4.648-653.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Yamada T., Tanimura A. Volatile nitrosamines in human blood before and after ingestion of a meal containing high concentrations of nitrate and secondary amines. Food Cosmet Toxicol. 1980 Jun;18(3):297–299. doi: 10.1016/0015-6264(80)90111-x. [DOI] [PubMed] [Google Scholar]



