Abstract
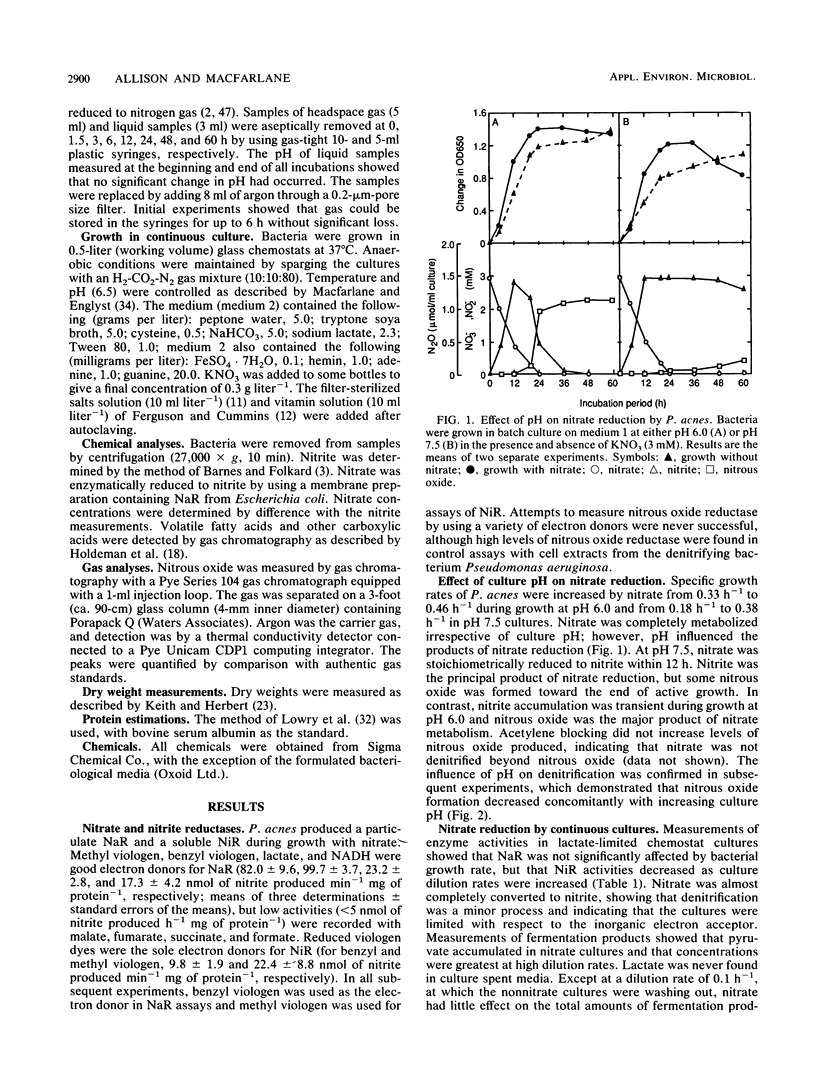
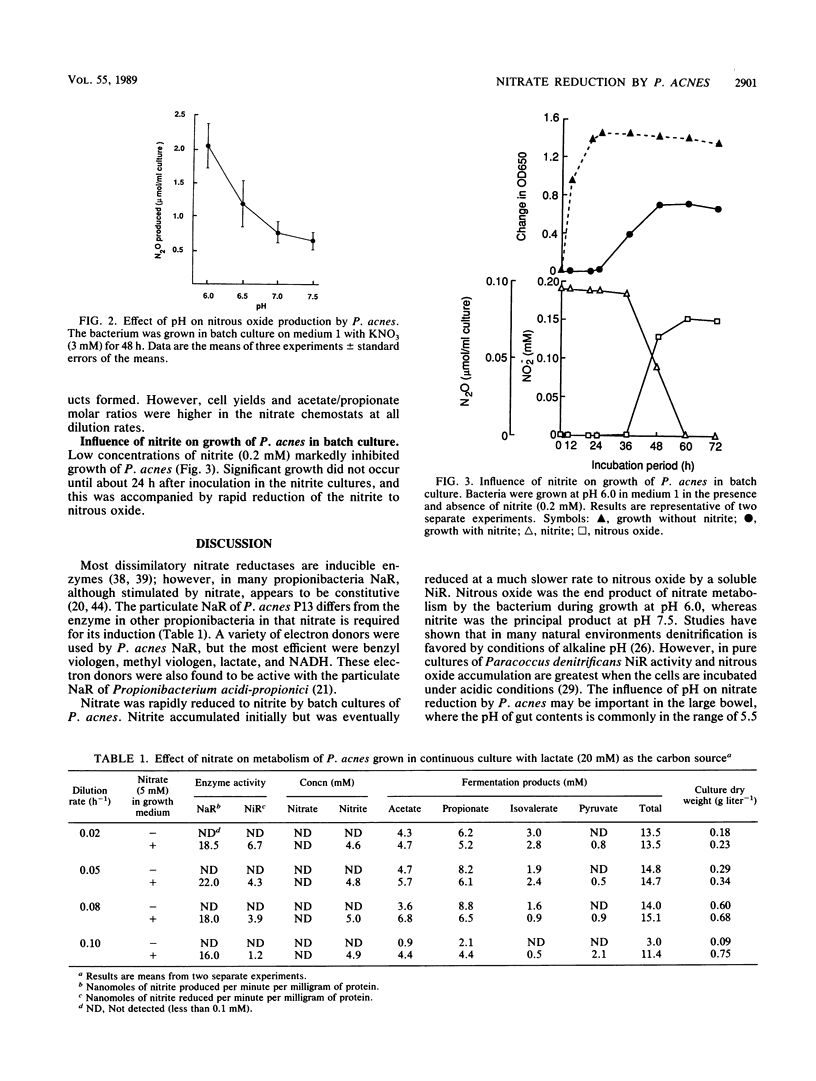
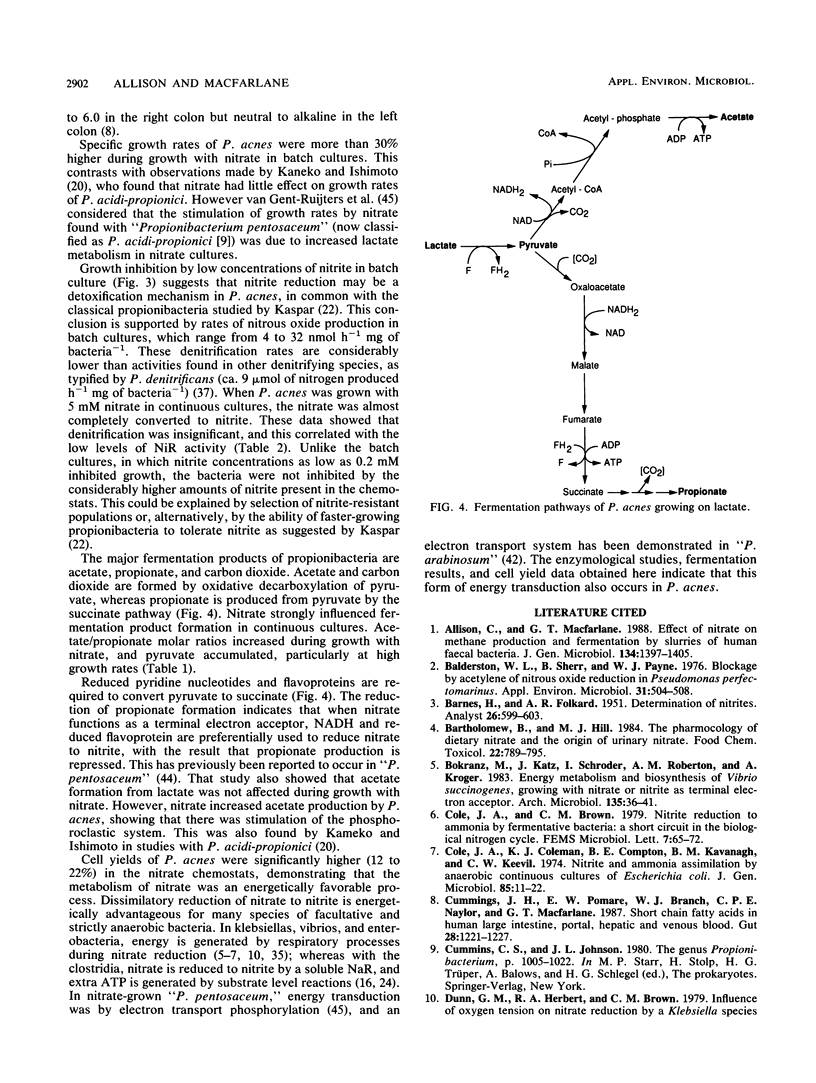
Propionibacterium acnes P13 was isolated from human feces. The bacterium produced a particulate nitrate reductase and a soluble nitrite reductase when grown with nitrate or nitrite. Reduced viologen dyes were the preferred electron donors for both enzymes. Nitrous oxide reductase was never detected. Specific growth rates were increased by nitrate during growth in batch culture. Culture pH strongly influenced the products of dissimilatory nitrate reduction. Nitrate was principally converted to nitrite at alkaline pH, whereas nitrous oxide was the major product of nitrate reduction when the bacteria were grown at pH 6.0. Growth yields were increased by nitrate in electron acceptor-limited chemostats, where nitrate was reduced to nitrite, showing that dissimilatory nitrate reduction was an energetically favorable process in P. acnes. Nitrate had little effect on the amounts of fermentation products formed, but molar ratios of acetate to propionate were higher in the nitrate chemostats. Low concentrations of nitrite (ca. 0.2 mM) inhibited growth of P. acnes in batch culture. The nitrite was slowly reduced to nitrous oxide, enabling growth to occur, suggesting that denitrification functions as a detoxification mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison C., Macfarlane G. T. Effect of nitrate on methane production and fermentation by slurries of human faecal bacteria. J Gen Microbiol. 1988 Jun;134(6):1397–1405. doi: 10.1099/00221287-134-6-1397. [DOI] [PubMed] [Google Scholar]
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Hill M. J. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem Toxicol. 1984 Oct;22(10):789–795. doi: 10.1016/0278-6915(84)90116-9. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Coleman K. J., Compton B. E., Kavanagh B. M., Keevil C. W. Nitrite and ammonia assimilation by anaerobic continuous cultures of Escherichia coli. J Gen Microbiol. 1974 Nov;85(1):11–22. doi: 10.1099/00221287-85-1-11. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. M., Herbert R. A., Brown C. M. Influence of oxygen tension on nitrate reduction by a Klebsiella sp. growing in chemostat culture. J Gen Microbiol. 1979 Jun;112(2):379–383. doi: 10.1099/00221287-112-2-379. [DOI] [PubMed] [Google Scholar]
- Ferguson D. A., Jr, Cummins C. S. Nutritional requirements of anaerobic coryneforms. J Bacteriol. 1978 Sep;135(3):858–867. doi: 10.1128/jb.135.3.858-867.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent-Ruijters M. L., Meijere F. A., Vries W., Stouthamer A. H. Lactate metabolism in Propionibacterium pentosaceum growing with nitrate or oxygen as hydrogen acceptor. Antonie Van Leeuwenhoek. 1976;42(3):217–228. doi: 10.1007/BF00394118. [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Hall J. B. The physiological function of nitrate reduction in Clostridium perfringens. J Gen Microbiol. 1975 Mar;87(1):120–128. doi: 10.1099/00221287-87-1-120. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Ishimoto M. A study on nitrate reductase from Propionibacterium acidi-propionici. J Biochem. 1978 Jan;83(1):191–200. doi: 10.1093/oxfordjournals.jbchem.a131891. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Ishimoto M. Effect of nitrate reduction on metabolic products and growth of Propionibacterium acidi-propionici. Z Allg Mikrobiol. 1977;17(3):211–220. doi: 10.1002/jobm.3630170306. [DOI] [PubMed] [Google Scholar]
- Knight T. M., Forman D., Al-Dabbagh S. A., Doll R. Estimation of dietary intake of nitrate and nitrite in Great Britain. Food Chem Toxicol. 1987 Apr;25(4):277–285. doi: 10.1016/0278-6915(87)90123-2. [DOI] [PubMed] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982 Mar;46(1):43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- LOWE R. H., EVANS H. J. PREPARATION AND SOME PROPERTIES OF A SOLUBLE NITRATE REDUCTASE FROM RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Jun 1;85:377–389. doi: 10.1016/0926-6569(64)90301-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K., Greger J. L., Consaul J. R., Graham K. L., Chinn B. L. Nitrate, nitrite balance, and de novo synthesis of nitrate in humans consuming cured meats. Am J Clin Nutr. 1986 Aug;44(2):188–194. doi: 10.1093/ajcn/44.2.188. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Englyst H. N. Starch utilization by the human large intestinal microflora. J Appl Bacteriol. 1986 Mar;60(3):195–201. doi: 10.1111/j.1365-2672.1986.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Malik A. C., Reinbold G. W., Vedamuthu E. R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968 Nov;14(11):1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. 8. Some properties of the N2O-anaerobically grown cell. J Biochem. 1971 Jun;69(6):991–1001. doi: 10.1093/oxfordjournals.jbchem.a129572. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe B. C., Hall C., Roediger W. E. Nitrite and nitrate levels in ileostomy effluent: effect of dietary change. Br J Nutr. 1989 Mar;61(2):323–330. doi: 10.1079/bjn19890120. [DOI] [PubMed] [Google Scholar]
- Saul R. L., Kabir S. H., Cohen Z., Bruce W. R., Archer M. C. Reevaluation of nitrate and nitrite levels in the human intestine. Cancer Res. 1981 Jun;41(6):2280–2283. [PubMed] [Google Scholar]
- Sone N. The redox reactions in propionic acid fermantation. I. Occurrence and nature of an electron transfer system in Propionibacterium arabinosum. J Biochem. 1972 Jun;71(6):931–940. doi: 10.1093/oxfordjournals.jbchem.a129864. [DOI] [PubMed] [Google Scholar]
- Thayer J. R., Chasko J. H., Swartz L. A., Parks N. J. Gut reactions of radioactive nitrite after intratracheal administration in mice. Science. 1982 Jul 9;217(4555):151–153. doi: 10.1126/science.6211766. [DOI] [PubMed] [Google Scholar]
- Van Gent-Ruijters M. L., DeVries W., Southamer A. H. Influence of nitrate on fermentation pattern, molar growth yields and synthesis of cytochrome b in Propionibacterium pentosaceum. J Gen Microbiol. 1975 May;88(1):36–48. doi: 10.1099/00221287-88-1-36. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]



