Abstract
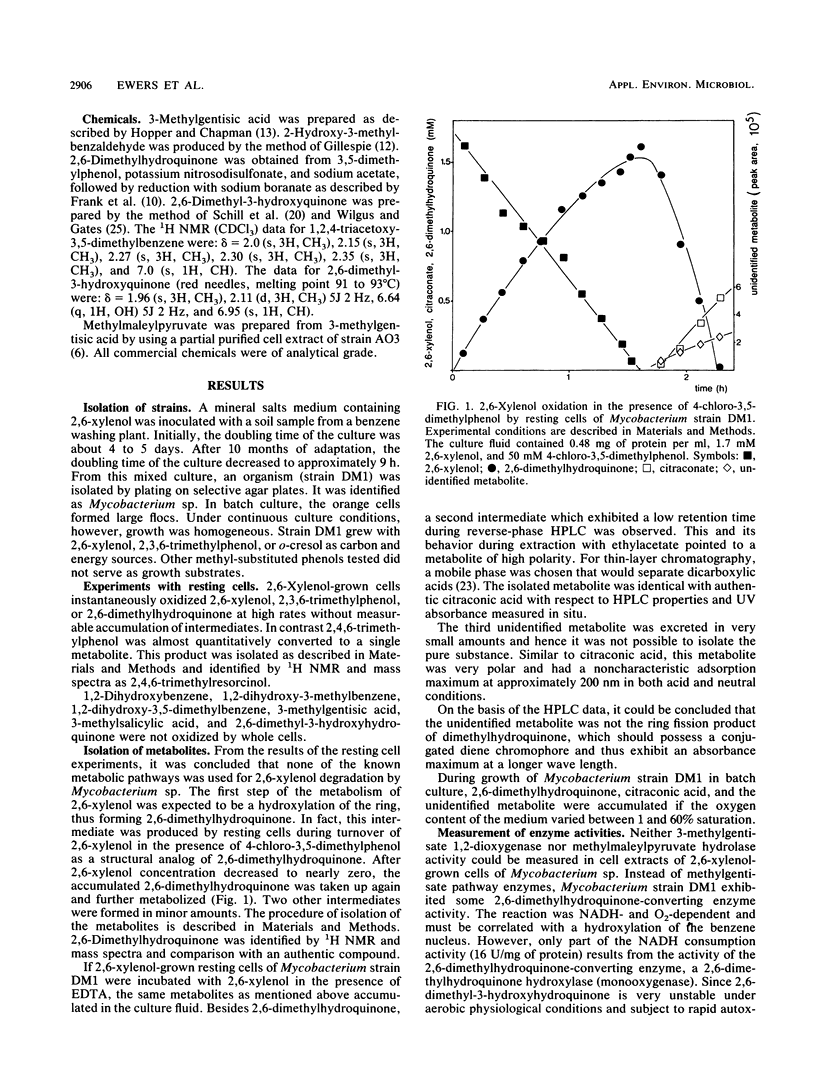
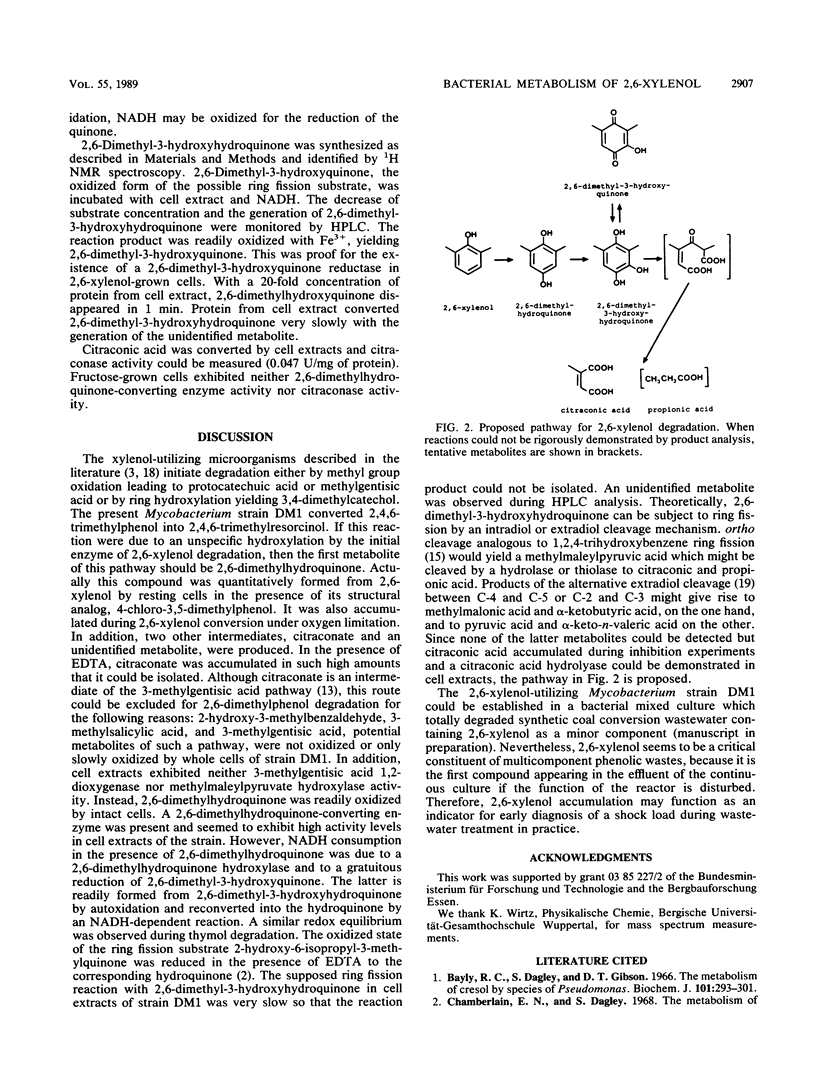
Strain DM1, a Mycobacterium sp. that utilizes 2,6-xylenol, 2,3,6-trimethylphenol, and o-cresol as sources of carbon and energy, was isolated. Intact cells of Mycobacterium strain DM1 grown with 2,6-xylenol cooxidized 2,4,6-trimethylphenol to 2,4,6-trimethylresorcinol. 4-Chloro-3,5-dimethylphenol prevents 2,6-xylenol from being totally degraded; it was quantitatively converted to 2,6-dimethylhydroquinone by resting cells. 2,6-Dimethylhydroquinone, citraconate, and an unidentified metabolite were detected as products of 2,6-xylenol oxidation in cells that were partially inactivated by EDTA. Under oxygen limitation, 2,6-dimethylhy-droquinone, citraconate, and an unidentified metabolite were released during 2,6-xylenol turnover by resting cells. Cell extracts of 2,6-xylenol-grown cells contained a 2,6-dimethylhydroquinone-converting enzyme. When supplemented with NADH, cell extracts catalyzed the reduction of 2,6-dimethyl-3-hydroxyquinone to 2,6-dimethyl-3-hydroxyhydroquinone. Since a citraconase was also demonstrated in cell extracts, a new metabolic pathway with 2,6-dimethyl-3-hydroxyhydroquinone as the ring fission substrate is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J., Hopper D. J. The bacterial metabolism of 2,4-xylenol. Biochem J. 1968 Dec;110(3):491–498. doi: 10.1042/bj1100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Phenol and benzoate metabolism by Pseudomonas putida: regulation of tangential pathways. J Bacteriol. 1969 Nov;100(2):869–877. doi: 10.1128/jb.100.2.869-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J. Gentisic acid and its 3- and 4-methyl-substituted homologoues as intermediates in the bacterial degradation of m-cresol, 3,5-xylenol and 2,5-xylenol. Biochem J. 1971 Mar;122(1):19–28. doi: 10.1042/bj1220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Kemp P. D. Regulation of enzymes of the 3,5-xylenol-degradative pathway in Pseudomonas putida: evidence for a plasmid. J Bacteriol. 1980 Apr;142(1):21–26. doi: 10.1128/jb.142.1.21-26.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. L., Bayly R. C. Evidence for isofunctional enzymes used in m-cresol and 2,5-xylenol degradation via the gentisate pathway in Pseudomonas alcaligenes. J Bacteriol. 1980 Jul;143(1):59–69. doi: 10.1128/jb.143.1.59-69.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- TABAK H. H., CHAMBERS C. W., KABLER P. W. MICROBIAL METABOLISM OF AROMATIC COMPOUNDS. I. DECOMPOSITION OF PHENOLIC COMPOUNDS AND AROMATIC HYDROCARBONS BY PHENOL-ADAPTED BACTERIA. J Bacteriol. 1964 Apr;87:910–919. doi: 10.1128/jb.87.4.910-919.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



