Abstract
Filamentous bacteria, identified as members of the genus Beggiatoa by gliding motility and internal globules of elemental sulfur, occur in massive aggregations at the deep-sea hydrothermal vents of the Guaymas Basin, Gulf of California. Cell aggregates covering the surface of sulfide-emanating sediments and rock chimneys were collected by DS R/V Alvin and subjected to shipboard and laboratory experiments. Each sample collected contained one to three discrete width classes of this organism usually accompanied by a small number of “flexibacteria” (width, 1.5 to 4 μm). The average widths of the Beggiatoa classes were 24 to 32, 40 to 42, and 116 to 122 μm. As indicated by electron microscopy and cell volume/protein ratios, the dominant bacteria are hollow cells, i.e., a thin layer of cytoplasm surrounding a large central liquid vacuole. Activities of Calvin-cycle enzymes indicated that at least two of the classes collected possess autotrophic potential. Judging from temperature dependence of enzyme activities and whole-cell CO2 incorporation, the widest cells were mesophiles. The narrowest Beggiatoa sp. was either moderately thermophilic or mesophilic with unusually thermotolerant enzymes. This was consistent with its occurrence on the flanks of hot smoker chimneys with highly variable exit temperatures. In situ CO2 fixation rates, sulfide stimulation of incorporation, and autoradiographic studies suggest that these Beggiatoa spp. contribute significantly as lithoautrophic primary producers to the Guaymas Basin vent ecosystems.
Full text
PDF


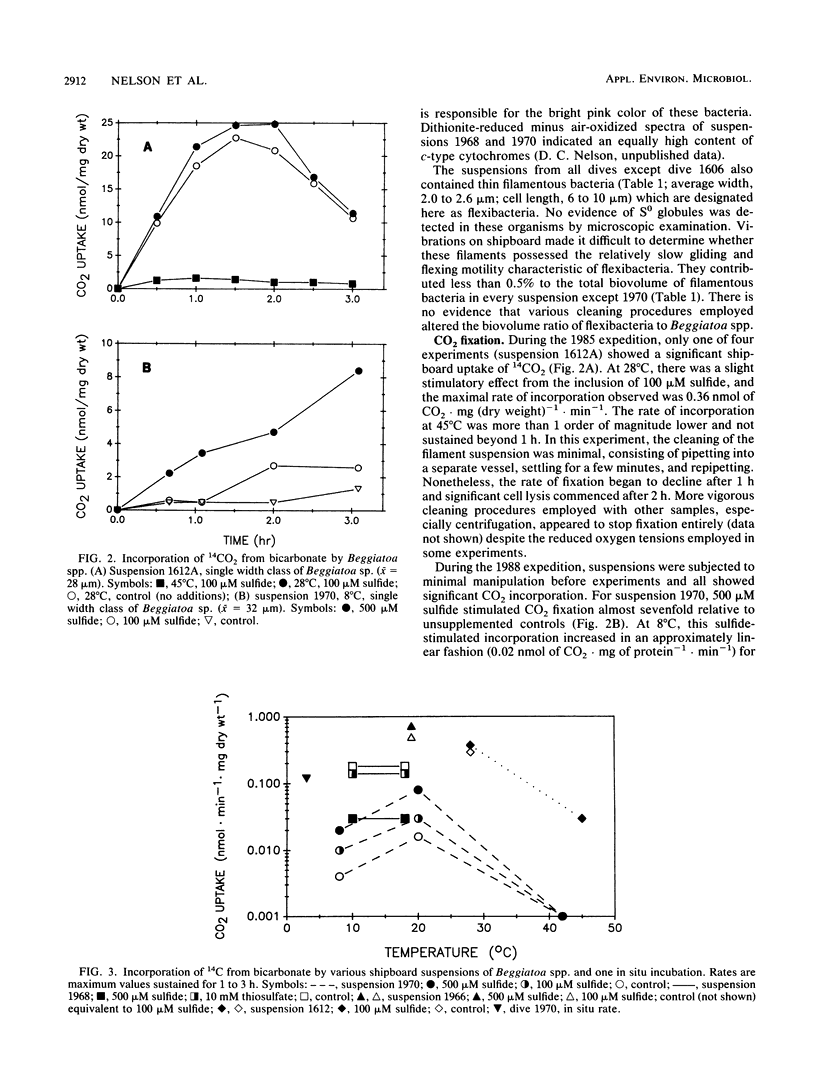




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Science. 1985 Oct 11;230(4722):132–138. doi: 10.1126/science.230.4722.132. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O., Molyneaux S. J., Langworthy T. A. Extremely thermophilic fermentative archaebacteria of the genus desulfurococcus from deep-sea hydrothermal vents. Appl Environ Microbiol. 1988 May;54(5):1203–1209. doi: 10.1128/aem.54.5.1203-1209.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981 Feb;41(2):528–538. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P. Colorless Sulfur Bacteria, Beggiatoa spp. and Thiovulum spp., in O(2) and H(2)S Microgradients. Appl Environ Microbiol. 1983 Apr;45(4):1261–1270. doi: 10.1128/aem.45.4.1261-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J. M., Strohl W. R. Beggiatoa, Thiothrix, and Thioploca. Annu Rev Microbiol. 1983;37:341–367. doi: 10.1146/annurev.mi.37.100183.002013. [DOI] [PubMed] [Google Scholar]
- Møller M. M., Nielsen L. P., Jørgensen B. B. Oxygen Responses and Mat Formation by Beggiatoa spp. Appl Environ Microbiol. 1985 Aug;50(2):373–382. doi: 10.1128/aem.50.2.373-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):140–154. doi: 10.1128/jb.147.1.140-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Jørgensen B. B., Revsbech N. P. Growth Pattern and Yield of a Chemoautotrophic Beggiatoa sp. in Oxygen-Sulfide Microgradients. Appl Environ Microbiol. 1986 Aug;52(2):225–233. doi: 10.1128/aem.52.2.225-233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]