Abstract
In this follow-up of a randomized placebo-controlled clinical trial of nicotine replacement transdermal patch for smoking cessation, 741 smokers of European ancestry who were randomized to receive active patch or placebo patch were genotyped for the serotonin transporter gene-linked polymorphic region. The study setting was a primary care research network in Oxfordshire, United Kingdom. The primary outcome measures were biochemically verified sustained abstinence from cigarette smoking at end of treatment and 24-week follow-up. The main effect of genotype was not associated with sustained abstinence from smoking at either end of treatment (SL: p=.33; SS: p=.81) or 24-week follow-up (SL: p=.05; SS: p=.21), and we found no evidence for a genotype×treatment interaction effect. In summary, despite the theoretically important contribution of serotonin neurotransmission to smoking cessation, the serotonin transporter gene was not associated with treatment response to nicotine patch for smoking cessation in this primary care–based trial.
Introduction
The serotonin (5-HT) system is implicated in the pathogenesis of multiple neuropsychiatric disorders and has received considerable attention in attempts to understand the molecular determinants of smoking (Veenstra-VanderWeele, Anderson, & Cook, 2000). Twin studies have demonstrated the role of genetic factors in the etiology of nicotine dependence (Li, Cheng, Ma, & Swan, 2003), and as focus turns toward the molecular genetic investigation of smoking-related phenotypes, serotonergic genes are biologically plausible candidates for evaluating genetic influences on smoking cessation.
The serotonin transporter gene (5-HTT) encodes a transmembrane transporter responsible for reuptake of serotonin at the synapse, and regulates the magnitude and duration of serotonergic signaling (Heils, Mossner, & Lesch, 1997; Heils et al., 1996; Lesch et al., 1994). A functional polymorphism in the promoter region (a 44 base-pair insertion/deletion polymorphism located approximately 1,000 base-pairs [bp] from the transcription initiation site) of the 5-HTT gene results in a polymorphism known as the serotonin transporter gene-linked polymorphic region (5-HTTLPR). In addition to the 5-HTTLPR, there are three additional variants in untranslated regions of the 5-HTT gene: (a) An approximately 380-bp deletion between the 5-HTTLPR and the transcription start site, which may affect gene expression (Flattem & Blakely, 2000; Lesch et al., 1999), (b) a VNTR within intron 2 associated with schizophrenia (Fan & Sklar, 2005) but not with either bipolar or unipolar depression (Lasky-Su, Faraone, Glatt, & Tsuang, 2005), and (c) a G/T polymorphism in the 3′ untranslated region (3′ UTR; Battersby et al., 1999; Lesch, Wolozin, Estler, Murphy, & Riederer, 1993), which appears to be associated with attention-deficit/hyperactivity disorder (Curran, Purcell, Craig, Asherson, & Sham, 2005; Kent et al., 2002). In all, there are more than 25 single nucleotide polymorphisms (SNPs), of which 16 are synonymous (amino acid changing) SNPs (Murphy, Lerner, Rudnick, & Lesch, 2004).
The 5-HTTLPR is known to be associated with altered serotonin activity. The short (S) form of this polymorphism is associated with decreased transcriptional activity compared with the long (L) form (Heils et al., 1996). In addition, S allele cells (SS or SL genotypes) have been shown to take up less serotonin from the medium than LL genotype cells in postmortem brain (Little et al., 1998), cultured lymphoblasts (Lesch et al., 1996), and cloned fusion experiments (Heils et al., 1996). Furthermore, a recent positron emission tomography study demonstrated reduced 5-HT1A receptor binding potential in the brains of S allele carriers (David et al., 2005). Preclinical research has also demonstrated that the S allele is associated with lower binding potential to the 5-HT1A receptor, which is implicated in nicotine-induced behavioral sensitization (Olausson, Engel, & Soderpalm, 1999). The S allele has also been reported to be associated with anxiety-related traits such as neuroticism and harm avoidance (Munafò et al., 2003; Sen, Burmeister, & Ghosh, 2004), whereas the L allele has been associated with obsessive-compulsive disorder (Bengel et al., 1999) and completed suicide (Du et al., 1999). Thus the 5-HTTLPR is a candidate gene of interest for the investigation of genetic influences on human behavior (Murphy et al., 2004).
The relationship between candidate genes and cigarette smoking has been studied widely, but despite a large number of association studies, a recent meta-analysis concluded that the findings remain somewhat equivocal (Munafò, Clark, Johnstone, Murphy, & Walton, 2004). In this analysis, however, the only candidate genes for which there was evidence of association with smoking behavior were the 5-HTT and cytochrome P450 2A6 genes. Moreover, the one dimension of smoking behavior in which the 5-HTTLPR polymorphism demonstrated a significant association was smoking cessation (Munafò, Clark et al., 2004), with the S allele associated with reduced likelihood of being an ex-smoker.
In this study we report the effects of 5-HTTLPR genotype on smoking cessation and response to nicotine replacement therapy (NRT) in participants in a randomized placebo-controlled trial of transdermal nicotine patch conducted in a large general practice research network in the United Kingdom. To date, two pharmacogenetic trials of NRT have been published in two study populations in the United Kingdom—the present sample (Johnstone et al., 2004; Yudkin et al., 2004)—and in the United States (Dahl et al., 2006; Lerman et al., 2004; Lerman et al., 2006; Malaiyandi et al., 2006). The Philadelphia-based study, which was an open-label trial that compared nicotine nasal spray to NRT patch (no placebo group) observed no association between the 5-HTTLPR and smoking cessation outcomes (Munafò et al., 2006). We based our hypotheses on evidence from our meta-analysis (Munafò, Clark et al., 2004), and from the data on the functional effects of the 5-HTTLPR polymorphism reviewed above. In particular, we predicted an effect of genotype on response to NRT, given the reported effects of 5-HTTLPR genotype on 5-HT1A binding reviewed above.
Our first hypothesis, therefore, was that there would be a main effect of 5-HTTLPR genotype, such that smokers carrying the S allele would demonstrate reduced sustained abstinence at 12-week end of treatment (EOT) and 24-week follow-up, compared with those with the LL genotype. We also hypothesized that smokers carrying the S allele would demonstrate greater relative NRT patch effectiveness compared to placebo for smoking cessation than those with the LL genotype.
Method
Participant recruitment
Participants in the original study included 1,686 patients from general practice surgeries in Oxfordshire, United Kingdom, who participated in a double-blind, randomized, placebo-controlled trial of the nicotine transdermal patch between June 1991 and March 1992 (the Patch Trial; Imperial Cancer Research Fund General Practice Research Group, 1993, 1994). The inclusion criteria for this study were that participants smoked at least 15 cigarettes/day and were aged 25–65 years. In 1999–2000, with approval from the local research ethics committee, and carried out through the Family Health Services Authority and each participant’s general practitioner, 1,532 were recontacted and invited to enter the study. Of the 1,686 participants enrolled in the Patch Trial, 154 subjects were unavailable because they could not be located (moved, emigrated, or untraceable) or were deceased. Invitation letters were sent to the remaining participants, and those interested in joining the study were given an appointment with a nurse at their general practitioner’s surgery, during which a short questionnaire was given and a 10-ml blood sample was collected. Blood samples were collected from 752 (49%) participants. The methods for recruitment, allocation, and randomization of the Patch Trial (Imperial Cancer Research Fund General Practice Research Group, 1993, 1994) and the follow-up at 8 years (Patch II Study; Johnstone et al., 2004; Yudkin et al., 2004) have been described comprehensively.
Ethical approval was obtained from the Anglia and Oxford Multi-centre Research Ethics Committee and from the 86 local research ethics committees covering the areas of residence of the patients.
Interventions
Participants were randomly assigned to wear active nicotine patches or placebo patches for 12 weeks by prior random allocation of study numbers to each intervention group and sequential allocation of a study number to patients on entry. Participants were assessed by a study nurse at 1, 4, 8, 12, 24, and 52 weeks. Active and placebo patches were identical as prepared by the manufacturer, and all investigators and patients were blinded to treatment allocation. The main outcome measure was reported abstinence.
Abstinence verification
Abstinence at 1, 4, and 8 weeks was confirmed by an expired carbon monoxide (CO) reading of 10 ppm or less, and at 12, 24, and 52 weeks by a salivary cotinine level of 20 ng/ml or less (89% of cases) or expired CO level of 10 ppm or less (11% of cases). Salivary cotinine was assayed by gas chromatography in the Department of Preventive Medicine at St. Bartholomew’s Medical College, London.
Genotyping
Blood samples were separated and frozen on the day of receipt, or stored overnight at 4°C. Plasma and buffy coat lymphocytes were stored at −80°C until required for analysis. Participants were genotyped in the Cancer Research UK General Practice Research Group Laboratory (Radcliffe Infirmary, Oxford, United Kingdom) using methods described previously (Lerman et al., 2000) and summarized briefly here. Oligonucleotide primers flanking the 5-HTTLPR from the 5-HTT gene (GenBank accession number X76753) were amplified using the polymerase chain reaction (PCR), and the amplified product was resolved by agarose gel electrophoresis resulting in ascertainment of one or more copies of a long (L: 528 bp) or short (S: 484 bp) VNTR. Two investigators blinded to subject smoking cessation outcomes reviewed PCR results independently.
Data analyses
Biochemically verified sustained abstinence, at the 12-week (EOT) and 24-week follow-ups, was the primary outcome measure. Participants lost to follow-up (154 [9%] out of 1,686 in the original trial population) were assumed to have relapsed to smoking and were coded as such in outcome analyses (i.e., intent-to-treat analyses). Separate models of outcome at the 12-week and 24-week follow-ups were generated within a logistic regression framework, because pharmacotherapy was available only during the treatment phase. Age, sex, and nicotine dependence score (Heatherton, Kozlowski, Frecker, & Fagcrström, 1991) were entered in the first step, treatment group (active patch, placebo patch) in the second step, and 5-HTTLPR genotype (LL, SL, SS) and a genotype×treatment group interaction term in the third step. For comparisons involving 5-HTTLPR genotype, LL was the reference group. An alpha level of .05 was maintained throughout the analysis.
Of the 741 participants in the final study population, 370 received active patch and 371 received placebo patch. The sample size was adequate to detect a risk ratio of 1.7 at 12 weeks and 1.8 at 24 weeks, with a power of 0.80, for a main effect of genotype on cessation (Figure 1).
Figure 1.
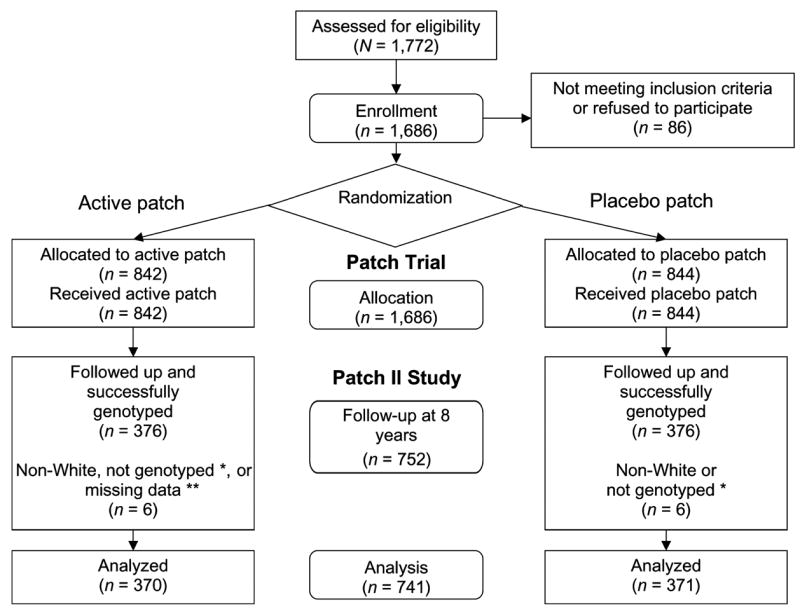
Flowchart describing progress of patients through the Patch II Study. Participants enrolled in a randomized, placebo-controlled trial of nicotine replacement patch (Patch Trial) were recontacted 8 years after the trial for genotyping (Patch II Study). Numbers of subjects at each point of contact are indicated in the figure. *One participant in each treatment group was not successfully genotyped. **Smoking outcome data was missing for one participant of Caucasian ancestry.
Results
Of the 750 participants who were successfully genotyped for the 5-HTTLPR polymorphism, 742 were of European ancestry. Data were missing on one participant, resulting in a final sample for analysis of 741 smokers (40% male) of European ancestry. The mean age of participants was 42 years 11 months (SD=9 years 11 months; range=25–65 years). 5-HTTLPR genotype frequencies by treatment group and abstinence at both 12-week (EOT) and 24-week follow-ups are presented in Table 1. Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium (p=.61).
Table 1.
5-HTTLPR genotype frequencies by treatment and abstinence at 12-week and 24-week follow-ups.
|
5-HTTLPR Genotype
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| SS
|
SL
|
LL
|
|||||||
| Active (n=72) | Placebo (n=59) | Combined (n=131) | Active (n=173) | Placebo (n=180) | Combined (n=353) | Active (n=125) | Placebo (n=132) | Combined (n=257) | |
| 12-Week | |||||||||
| Succeeded | 18 (25%) | 7 (12%) | 25 (19%) | 33 (19%) | 18 (10%) | 51 (14%) | 24 (19%) | 18 (14%) | 42 (16%) |
| Failed | 54 (75%) | 52 (88%) | 106 (81%) | 140 (81%) | 162 (90%) | 302 (86%) | 101 (81%) | 114 (86%) | 215 (84%) |
| 24-Week | |||||||||
| Succeeded | 12 (17%) | 4 (7%) | 16 (12%) | 22 (13%) | 12 (7%) | 34 (10%) | 23 (18%) | 18 (14%) | 41 (16%) |
| Failed | 60 (83%) | 55 (93%) | 115 (88%) | 151 (87%) | 168 (93%) | 319 (90%) | 102 (82%) | 114 (86%) | 216 (84%) |
Note. All values are numbers of subjects with percentages.
The main effect of treatment was not significant at either the 12-week (p=.18) or 24-week (p=.25) follow-up, although the odds ratio observed was compatible with an approximately 50% increased likelihood of successful cessation on active nicotine transdermal patch relative to placebo, as reported previously (Imperial Cancer Research Fund General Practice Research Group, 1993, 1994).
The main effect of 5-HTTLPR genotype was not associated with abstinence at either the 12-week (SL: p=.33; SS: p=.81) or 24-week (SL: p=.05; SS: p=.21) follow-up, and we found no evidence for a genotype×treatment interaction effect (Table 2). When participants with SS and SL alleles were grouped and compared with participants with LL genotypes, as has been performed by other investigators (David et al., 2005; Lerman et al., 2000; Munafò, Clark et al. 2004), these results did not change substantively.
Table 2.
Logistic regression models of abstinence at 12-week and 24-week follow-ups.
| 12-Week follow-up
|
24-Week follow-up
|
|||
|---|---|---|---|---|
| Variable | OR (95% CI) | p value | OR (95% CI) | p value |
| Age | 1.03 (1.01–1.05) | .009 | 1.03 (1.01–1.05) | .007 |
| Sex | 0.68 (0.46–1.02) | .065 | 0.79 (0.51–1.25) | .318 |
| Nicotine dependencea | 1.00 (0.96–1.05) | .864 | 0.98 (0.93–1.03) | .386 |
| Treatment | 1.59 (0.81–3.14) | .176 | 1.49 (0.75–2.96) | .251 |
| Genotypeb | ||||
| LL | 1.00 | 1.00 | ||
| SL | 0.71 (0.35–1.43) | .335 | 0.46 (0.21–1.00) | .049 |
| SS | 0.89 (0.35–2.29) | .809 | 0.48 (0.15–1.50) | .206 |
| Genotypeb × treatment | ||||
| LL | 1.00 | 1.00 | ||
| SL | 1.40 (0.56–3.50) | .473 | 1.44 (0.53–3.94) | .477 |
| SS | 1.39 (0.42–4.55) | .588 | 1.68 (0.42–6.74) | .464 |
Nicotine dependence severity assessed by the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Reference group is LL.
Age was positively associated with an increased likelihood of smoking cessation at the 12-week (p=.01) and 24-week (p=.01) follow-ups. All other effects were nonsignificant, although the effect of sex on abstinence at the 12-week follow-up approached statistical significance (p=.07), reflecting a trend for men to be more likely to be abstinent than women.
The full logistic regression models for abstinence at both the 12-week and 24-week follow-ups are presented in Table 2.
Additional outcomes such as withdrawal symptoms and adverse reactions to the patch were not included in this analysis and have been reported previously (Imperial Cancer Research Fund General Practice Research Group, 1993, 1994).
Discussion
The results of this study do not support the hypothesis that NRT patch effectiveness is greater in smokers with LL genotypes than with SS or SL genotypes at either the 12-week (EOT) or 24-week follow-up. We are not aware of any published studies examining NRT by 5-HTTLPR interactions for smoking cessation; therefore, we cannot say whether our results can be generalized to other populations.
Arguably, a weakness of the present study is its sample size. As is the case in most pharmacogenetic clinical trials for smoking cessation, a larger sample size would have permitted examination of whether the 5-HTTLPR interacted with other candidate genes or gender to affect treatment response to the nicotine patch (Cardon, Idury, Harris, Witte, & Elston, 2000; Munafò, Bradburn, Bowes, & David, 2004). However, the sample size was sufficient to detect clinically and statistically significant main effects of the 5-HTTLPR on smoking cessation and treatment response to the patch. Moreover, we demonstrated a significant interaction between dopaminergic genes (dopamine D2 and dopamine beta hydroxylase) and treatment in this study population with a similar sample size (Johnstone et al., 2004). Nevertheless, we regard it as important to publish nonsignificant results from genetic association studies, to facilitate future meta-analyses.
Another limitation of the study is that the 5-HTT gene has multiple polymorphisms, and at least two (intron 2 VNTR, 3′ UTR G/T) of these SNPs have been associated with behavioral phenotypes (Curran et al., 2005; Fan & Sklar, 2005; Kent et al., 2002; Lasky-Su et al., 2005). However, in both cases, no associations have been established with smoking phenotypes nor with 5-HT receptor function as in the case of the well-described 5-HTTLPR. In addition, in a recent association study, Hu and colleagues (2005) cite unpublished data suggesting that there are two subtypes of the 5-HTTLPR L allele and that only one of these subtypes, LA, is “high activity”. Thus the lack of subclassification of the 5-HTTLPR presents a potential confound in our analyses of the polymorphism and potential associations with treatment response to nicotine patch. However, no well-described published in vitro studies have demonstrated differences in transcription between 5-HTTLPR L subtypes.
A statistical trend was observed for a main effect of sex on sustained abstinence at 12 weeks, a finding consistent with emerging evidence for sex differences in smoking cessation. Human (Lee et al., 2005) and mouse (Li, Wichems, Heils, Lesch, & Murphy, 2000) studies suggest a moderating effect of sex on the relationship between the 5-HTT gene and 5-HT1A receptor expression. Therefore, either through analysis of larger pharmacogenetic studies or meta-analytic techniques, it will be worthwhile to examine whether any potential moderating influence of sex interacts significantly with the 5-HTTLPR to affect smoking cessation outcomes.
In conclusion, we found no evidence to suggest a main effect of 5-HTTLPR genotype on smoking cessation, or a moderating effect of genotype on NRT response compared with placebo. The 5-HTTLPR polymorphism is a potentially useful candidate gene for treatment response to selective serotonin-reuptake inhibitors (Lesch & Gutknecht, 2005). However, at least in this population of patients in a primary care setting in the United Kingdom, the 5-HTTLPR does not appear to be helpful for tailoring nicotine patch therapy for smoking cessation.
Acknowledgments
The authors thank Robyn Jacob and Maureen Proctor for assistance in genotyping; Kate Hey, Sarah Roberts, and Sarah Welch for data collection; Siân Griffiths for cotinine analysis; Patricia Yudkin and Bing Lu for statistical assistance; Professor Edith Sim. The study was funded by Cancer Research UK, which approved but had no involvement in planning the study. Nicotine patches and placebo patches were provided by Ciba-Geigy Pharmaceuticals. Personal funding for SPD was provided by career development awards from the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program and National Institute on Drug Abuse (NIDA)/National Institutes of Health (NIH) grant PHS 1 K08 DA14276-01A1, National Cancer Institute, NIDA, National Institute on Alcohol Abuse and Alcoholism grant PSOCA 84719.
Contributor Information
Sean P. David, Brown Medical School/Memorial Hospital of Rhode Island, Pawtucket, RI, and University of Oxford, United Kingdom
Marcus R. Munafò, University of Bristol, United Kingdom
Michael F. G. Murphy, Childhood Cancer Research Group, University of Oxford, United Kingdom
Robert T. Walton, Medical Research Council Laboratories, The Gambia.
Elaine C. Johnstone, University of Oxford, United Kingdom.
References
- Battersby S, Ogilvie AD, Blackwood DH, Shen S, Muqit MM, Muir WJ, Teague P, Goodwin GM, Harmar AJ. Presence of multiple functional polyadenylation signals and a single nucleotide polymorphism in the 3′ untranslated region of the human serotonin transporter gene. Journal of Neurochemistry. 1999;72:1384–1388. doi: 10.1046/j.1471-4159.1999.721384.x. [DOI] [PubMed] [Google Scholar]
- Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, Murphy DL. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Molecular Psychiatry. 1999;4:463–466. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Idury RM, Harris TJ, Witte JS, Elston RC. Testing drug response in the presence of genetic information: Sampling issues for clinical trials. Pharmacogenetics. 2000;10:503–510. doi: 10.1097/00008571-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Curran S, Purcell S, Craig I, Asherson P, Sham P. The serotonin transporter gene as a QTL for ADHD. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2005;134:42–47. doi: 10.1002/ajmg.b.30118. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Jepson C, Levenson R, Wileyto EP, Patterson F, Berrettini WH, Lerman C. Interaction between variation in the D2 dopamine receptor (DRD2) and the neuronal calcium sensor-1 (FREQ) genes in predicting response to nicotine replacement therapy for tobacco dependence. Pharmacogenomics Journal. 2006;6:194–199. doi: 10.1038/sj.tpj.6500358. [DOI] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafò MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1a receptor binding in humans. Journal of Neuroscience. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, Demeter E, Bakish D, Lapierre YD, Sotonyi P, Hrdina PD. Frequency of long allele in serotonin transporter gene is increased in depressed suicide victims. Biological Psychiatry. 1999;46:196–201. doi: 10.1016/s0006-3223(98)00376-x. [DOI] [PubMed] [Google Scholar]
- Fan JB, Sklar P. Meta-analysis reveals association between serotonin transporter gene stin2 VNTR polymorphism and schizophrenia. Molecular Psychiatry. 2005;10:928–938. 891. doi: 10.1038/sj.mp.4001690. [DOI] [PubMed] [Google Scholar]
- Flattem NL, Blakely RD. Modified structure of the human serotonin transporter promoter. Molecular Psychiatry. 2000;5:110–115. doi: 10.1038/sj.mp.4000585. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism—Basic research and clinical implications. Journal of Neural Transmission. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism, Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: Results of a randomised trial in general practice. British Medical Journal. 1993;306:1304–1308. doi: 10.1136/bmj.306.6888.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Randomised trial of nicotine patches in general practice: Results at one year. British Medical Journal. 1994;308:1476–1477. [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, Griffiths SE, Walton RT. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14:83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Kent L, Doerry U, Hardy E, Parmar R, Gingell K, Hawi Z, Kirley A, Lowe N, Fitzgerald M, Gill M, Craddock N. Evidence that variation at the serotonin transporter gene influences susceptibility to attention deficit hyperactivity disorder (ADHD): Analysis and pooled analysis. Molecular Psychiatry. 2002;7:908–912. doi: 10.1038/sj.mp.4001100. [DOI] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2005;133:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Lee M, Bailer UF, Frank GK, Henry SE, Meltzer CC, Price JC, Mathis CA, Putnam KT, Ferrell RE, Hariri AR, Kaye WH. Relationship of a 5-HT transporter functional polymorphism to 5-HT(1a) receptor binding in healthy women. Molecular Psychiatry. 2005;10:715–716. doi: 10.1038/sj.mp.4001680. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Boyd NR, Shields PG. Interacting effects of the serotonin transporter gene and neuroticism in smoking practices and nicotine dependence. Molecular Psychiatry. 2000;5:189–192. doi: 10.1038/sj.mp.4000672. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, Kaufmann V, Restine S, Hawk L, Niaura R, Berrettini W. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: Results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH. The functional mu opioid receptor (OPRM1) asn40asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics Journal. 2004;4:184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. Journal of Neural Transmission: Genetics Section. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Pharmacogenetics of the serotonin transporter. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2005;29:1062–1073. doi: 10.1016/j.pnpbp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Jatzke S, Meyer J, Stober G, Okladnova O, Mossner R, Riederer P. Mosaicism for a serotonin transporter gene promoter-associated deletion: Decreased recombination in depression. Journal of Neural Transmission. 1999;106:1223–1230. doi: 10.1007/s007020050236. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Estler HC, Murphy DL, Riederer P. Isolation of a cDNA encoding the human brain serotonin transporter. Journal of Neural Transmission: Genetics Section. 1993;91:67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not g-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: Gender and brain region differences. Journal of Neuroscience. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. American Journal of Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Molecular Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Bradburn M, Bowes L, David S. Investigating subgroups in smoking cessation treatment response. Nicotine & Tobacco Research. 2004;6:865–867. [Google Scholar]
- Munafò MR, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Clark TG, Moore LR, Payne E, Walton R, Flint J. Genetic polymorphisms and personality in healthy adults: A systematic review and meta-analysis. Molecular Psychiatry. 2003;8:471–484. doi: 10.1038/sj.mp.4001326. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Wileyto EP, Shields PG, Elliot KM, Lerman C. Lack of association of 5-HTTLPR genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:398–400. doi: 10.1158/1055-9965.EPI-05-0648. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Molecular Interventions. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Olausson P, Engel JA, Soderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology. 1999;142:111–119. doi: 10.1007/s002130050869. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: Initial studies and future directions. European Journal of Pharmacology. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- Yudkin P, Munafò M, Hey K, Roberts S, Welch S, Johnstone E, Murphy M, Griffiths S, Walton R. Effectiveness of nicotine patches in relation to genotype in women versus men: Randomised controlled trial. British Medical Journal. 2004;328:989–990. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]


