Abstract
Background:
Spinal cord injury (SCI) has been found to affect the physiology of the gastrointestinal tract. Changes in gastric motility occur in tetraplegia because of dissociation of antral and duodenal motility. Among individuals with high-level tetraplegia, antral quiescence has been hypothesized as a manifestation of autonomic dysreflexia after surgery. This case series shows the issues with gastric hypomotility after gastrointestinal surgery in tetraplegic patients with tetraplegia, including management strategies.
Objective:
To report 3 patients with complete high cervical SCI who developed gastroparesis after abdominal surgery and discuss the effect of autonomic dysfunction on gastric motility.
Methods:
Retrospective chart review of 3 cases.
Results:
Gastroparesis occurred after abdominal surgery in 3 patients with C4 American Spinal Injury Association (ASIA) A tetraplegia and seemed to be a sign of autonomic hyperreflexia caused by postoperative pain. Management was challenging because it consisted of balancing of appropriate pain medication and dealing with absorption issues and dysmotility. Often gastric motility agents were not effective in improving gastric emptying. However, increased use of pain medication improved gastric emptying, which supports the hypothesis that this issue represents gastric dysfunction from autonomic hyperreflexia.
Conclusions:
In persons with complete cervical SCI who have undergone abdominal surgery, postoperative gastroparesis can be a manifestation of pain. This may occur as the excessive sympathetic response from autonomic hyperreflexia inhibits distal antral activity. Thus, treatment of postoperative gastroparesis should focus on improved pain control to decrease excessive splanchnic sympathetic output and circulating norepinephrine.
Keywords: Autonomic hyperreflexia, Gastroparesis, Spinal cord injuries, Tetraplegia, Pain management, Pain, postoperative, Fentanyl, Tegaserod
INTRODUCTION
Spinal cord injury (SCI) has been found to affect the physiology of the gastrointestinal tract, causing problems that include delayed gastric emptying, altered gastric secretion caused by autonomic dysfunction, and abnormal colonic myoenteric activity (1–11). However, there are few studies on postoperative gastric emptying in SCI or the effect of autonomic hyperreflexia on gastrointestinal motility.
Studies in persons with SCI often show reduced gastric emptying at baseline. Kao et al (12) did radionuclide imaging and found that SCI can cause significant prolonged gastric emptying of a solid meal, especially in female patients and patients with high level of injury. However, Zhang et al (13) studied the various emptying rates between solids and liquids and found essentially normal rates of gastric emptying of liquids and solids. This may be partially explained by the work of Gondim et al (14), who showed less inhibition of gastric emptying if large bowel emptying has occurred. Enck et al (15) also found that reduced gastric emptying may be attributable to a cologastric inhibitory reflex triggered by delayed colonic emptying.
Studies have shown that the gastric pacemaker potential no longer originates in the antrum in most individuals with tetraplegia (8,16). This adds to the decreased gastric emptying often seen in chronic tetraplegia. Segal et al (9) showed a biphasic pattern of gastric emptying in which an initial 20- to 30-minute phase of emptying was followed by a delayed second phase. Lu and Chen (10) showed electrogastrogram (EGG) abnormalities in gastric motor function in 12 patients with complete cervical SCI.
Fealey et al (7) reported on the effect of chronic SCI and autonomic dysreflexia on human gastrointestinal motility and gastric emptying. They reported that the changes in gastric motility occur in tetraplegia but do not occur in paraplegia. In tetraplegia, dissociation of antral and duodenal motility occurred. One subject had prominent recurrent autonomic hyperreflexia and was noted to have marked antral hypomotility, which was highly associated with the degree of reflex vascular hypertension. In contrast, duodenal motility was unaffected by autonomic hyperreflexia. The authors hypothesized that excessive splanchnic sympathetic output may delay gastric emptying by inhibiting distal antral activity.
Sympathetic stimulation to the gut is considered in general to be inhibitory (17). Hermann et al (18) showed that α-1 agonists suppress gastric motility. In addition, the α-2 receptor is the main inhibitor of the migrating motor complex in the gut and seems to be the primary receptor responsible for ileus (17). During autonomic dysreflexia (AD), elevating circulating catecholamine levels are found (19–22). Thus, it is possible that autonomic dysreflexia is associated with delayed gastric emptying by increasing the sympathetic input to the gut.
This case series reports 3 patients with C4 American Spinal Injury Association (ASIA) A tetraplegia who underwent elective abdominal surgery (for either bowel or bladder problems). All 3 had postoperative gastroparesis and autonomic hyperreflexia and improvement when treated with pain medications. However, gastric atony occurred in 2 of the 3 patients, requiring percutaneous gastrostomy tube placement for venting. The discussion focuses on the significance of these observations and recommendations for future management of patients with high tetraplegia who require abdominal surgery.
CASE REPORTS
Case 1
A 41-year-old woman with a history of C4 ASIA A SCI from a diving accident more than 2 decades ago was admitted for elective total colectomy caused by severe constipation affecting bowel program and care. Work-up was done before admission. She had worsening constipation with increasing reliance on large doses of laxatives. The bowel program frequently caused autonomic hyperreflexia. Transit study showed markers stopping at the terminal ileum. She underwent total proctocolectomy with end-ileostomy.
Postoperative pain was controlled with continuous intravenous (IV) morphine, titrated to symptoms of dysreflexia (headache and increased blood pressure). The morphine pump was gradually weaned off, and a fentanyl transdermal patch was started for pain control at 50 μg/h. She developed respiratory secretions and needed respiratory treatments. The fentanyl transdermal patch dosage was lowered to 25 μg/h on postoperative day (POD) 5. On POD 9, she was weaned off the IV morphine. Abdominal distention was noted with minimal stool output through the ileostomy. At POD 11, the patient complained of abdominal pain, and IV morphine was restarted at 3 mg/h. On POD 12, sustained-release oxycodone 10 mg by mouth twice a day was added and titrated up to 40 mg twice a day while the IV morphine was titrated down.
When all of the IV pain medication was discontinued on POD 14, her abdomen distended again, and poor stool output was noted. This was also associated with nausea, which was treated with Phenergan. There were episodes of autonomic hyperreflexia at POD 16 associated with a distended, firm abdomen. Other causes of autonomic dysreflexia were ruled out including bladder, skin, and infection. Postoperative pain was contributing to abdominal distention. Abdominal films confirmed distended stomach and small bowel. IV morphine was restarted, and the fentanyl patch dosage was increased. As pain control improved, dysreflexia resolved, abdominal girth decreased, and stool output improved. On POD 21, the patient was discharged home on fentanyl patch (Duragesic, Raritan, New Jersey) 75 mg/h every 72 hours and oxycodone elixir 40 mg every 6 hours as needed.
Case 2
A 30-year-old man with C4 ASIA A SCI caused by a spinal hematoma was admitted for elective hemicolectomy because of severe constipation leading to increasing spasticity. Work-up was done before admission. He had increasing abdominal distention and spasticity, with poor results from his bowel programs. Transit study showed decrease distal colon motility with large redundant loop of sigmoid. He underwent extended left colectomy with mobilization of splenic flexure and reanastomosis.
Postoperatively, he was placed on IV morphine. At this point, his abdomen was soft and not distended. As he continued on the IV morphine, no abdominal spasms were noted. On POD 4, he was treated for a methicillin-resistant Staphylococcus aureus urinary tract infection. Bowel movements (with suppository and digital stimulation) returned, and IV morphine was decreased. By POD 6, the patient was off the IV morphine. However, he started having autonomic dysreflexic episodes (Tables 1 and 2) and emesis with increasing abdominal distention. Because the patient was off IV morphine, postoperative pain caused autonomic dysreflexia, which clinically presented as abdominal distention. Other causes of autonomic dysreflexia were ruled out. A rectal tube was inserted with 50-mL output. A radiograph showed a dilated stomach. Nasogastric tube (NGT) output increased to 1,250 mL over 1 shift. Immediate IV morphine was given, and as pain resolved, there was decreased NGT output and improved blood pressures. To improve gastric motility, erythromycin was started. Despite oral narcotics, episodes of autonomic dysreflexia went on for the next week. At this point, the surgical team was reluctant to use aggressive pain management because these narcotic pain medications were thought to decrease gastric motility.
Table 1.
Blood Pressure and Heart Rate Record
Table 2.
Nasogastric Output and Relation With Abdominal Distention
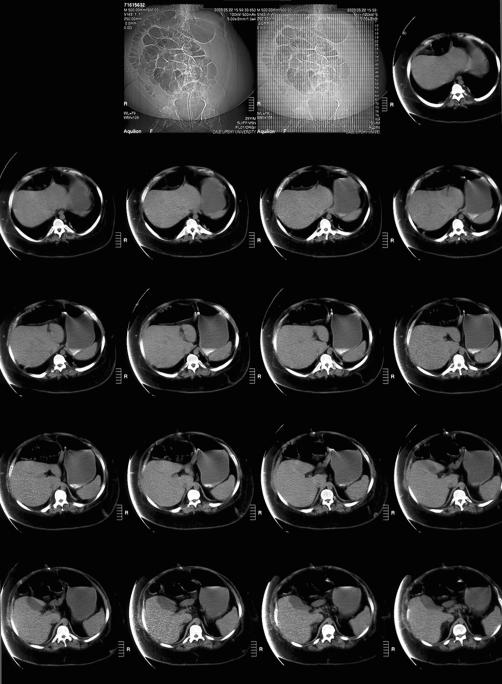
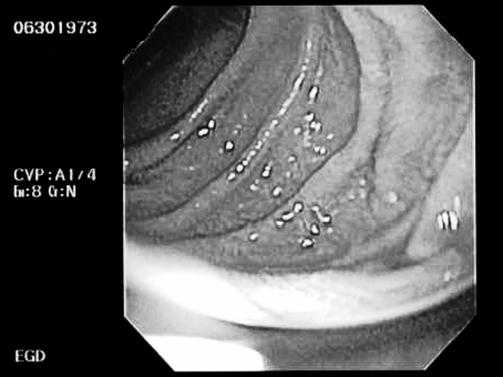
On POD 14, the patient was placed on total parenteral nutrition (TPN) because he was unable to take food by mouth. There was a question as to whether oral pain medications were being absorbed properly because of the gastric emptying problems and he continued to show signs of pain with abdominal spasms. Thus, he was restarted on IV morphine. After 1 day of IV morphine, his NGT output decreased, and he had large bowel movements. A trial of regular diet was implemented, but as the diet was advanced, he developed nausea and emesis again. Autonomic hyperreflexia episodes returned. Tegaserod 6 mg twice daily was tried to promote gastric emptying as narcotics were decreased. However, again, the patient had increased abdominal spasms and increase in abdominal girth. Computed tomography (CT) of the abdomen (Figure 1) was done and showed a markedly distended abdomen. Esophasogogastroduodenoscopy (EGD) showed marked gastric distention and gastric atony (Figure 2). A percutaneous gastrojejunostomy (PEG-J) tube was placed, and the patient was started on a transdermal fentanyl patch. He was observed for the next few days and was able to begin oral feedings. His abdomen remained soft, and no nausea or emesis occurred. Occasional abdominal distension developed but resolved when air was vented from the stomach through the PEG-J. On POD 42, he was discharged home with a transdermal fentanyl patch, tolerating a regular diet.
Figure 1. CT abdomen with marked abdominal distention (Case 2).
Figure 2. Esophagogastroduodenoscopy image (Case 2).
Case 3
A 31-year-old man with C4 ASIA A SCI caused by a gunshot wound more than 10 years ago was admitted for elective colectomy because of failure of right-sided colostomy for bowel management and for cystectomy caused by chronic cystitis. Past medical history was also notable for kidney and bladder stones, with chronic cystitis, and hemicolectomy with right-sided colostomy. He has a chronic indwelling suprapubic tube for bladder management. Preoperative radiographs showed a non-distended abdomen. He underwent total colectomy, partial cystectomy, and ileal loop with ileostomy.
Postoperatively, he was on IV morphine. He complained of abdominal discomfort and had nausea and vomiting, decreased bowel sounds, and a distended abdomen. Mildly elevated blood pressure was noted. The IV morphine dose was increased, which decreased the blood pressure, relieved the abdominal symptoms, and returned bowel sounds. He had a resolving ileus, but delirium developed, which was thought to be secondary to pain medication; thus, the IV morphine was decreased. As IV pain medication was tapered, his stomach gradually increased in girth, nausea ensued, and an orogastric (OG) tube output increased to 1,250 mL during one shift. Abdominal discomfort returned; thus, the IV morphine was increased, which improved the nausea. Then, the OG tube was discontinued.
The following day, the patient developed nausea and vomiting, and the OG tube was replaced, returning 3,500 mL. A transdermal fentanyl patch was started, as was tegaserod, with some relief of symptoms. Over the next few days, the IV morphine was tapered and discontinued. The patient again developed confusion, thought to be secondary to the transdermal fentanyl, so this was discontinued. This was followed by progressive abdominal distension. For the next few days, it was difficult to adjust pain medications and control dysreflexic episodes and abdominal distention. In addition, the patient developed fever caused by Klebsiella bacteremia and Candidemia.
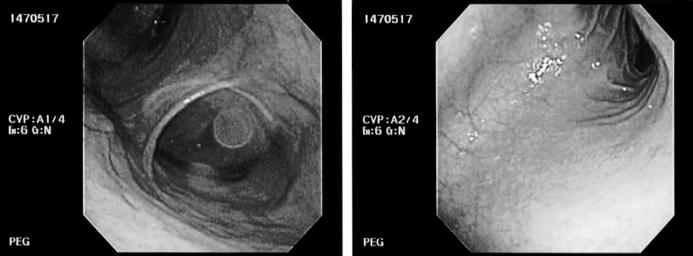
On POD 21, he underwent revision of the uretero-intestinal anastomosis and ileal conduit. Postoperatively, preventing gastroparesis and ileus with adequate pain and blood pressure management was a challenge. One week after the second surgery, EGD showed severe gastric atony (Figure 3), and a percutaneous endoscopic gastrostomy (PEG) tube placement was done because of delayed gastric emptying with persistent nausea and vomiting. The PEG tube was used to vent the stomach as the patient's abdomen became distended. Eventually, he was restarted on pureed diet and gradually tolerated the diet well. He was discharged home 1 week after PEG placement.
Figure 3. Esophagogastroduodenoscopy image (Case 3).
DISCUSSION
This series shows an interesting phenomenon, which supports the hypothesis of Fealey et al (7) that autonomic hyperreflexia affects gastric emptying in persons with SCI. In this series, 3 patients with complete cervical SCI had postoperative gastroparesis after abdominal surgery, in association with elevated blood pressures (Table 1). In all cases, increased narcotics seemed to improve gastric motility, in addition to decreasing blood pressure (Table 2). Our assumption was that these patients have postoperative pain, which is universal in patients after abdominal surgery. The elevated blood pressures in these patients confirmed that they were experiencing a painful stimulus below the level of their injury. Treatment with narcotics reduced the blood pressure by treating the pain. The unexpected finding was that the degree of gastroparesis correlated with the increased blood pressure, and narcotics actually improved gastric emptying (rather than delaying it as one would fear).
There is acute stress placed on gastrointestinal motility associated with surgery. Acute ileus is common after abdominal surgeries (23,24). However, in the first 2 cases, ileus did not appear acutely. Rather, the patients had gastroparesis that occurred on the second week postoperatively, coinciding with the tapering of intravenous pain medication and just as oral pain medications were added. In case 3, the patient did have postoperative ileus but also had a more complicated medical course. However, his abdominal distension may also have been caused in part by autonomic hyperreflexia, because it correlated with high blood pressure and improved with IV morphine.
Often initial management by the surgeons was to decrease the narcotics, because of the gastric emptying delay these can cause. The decreased gastric emptying was confirmed by CT and EGD in our case series (as well as by high gastric output using a NG or OG tube). In all cases, the reduced gastric emptying was accompanied by increases in blood pressure consistent with autonomic dysreflexia.
In this case series, patients were managed primarily with IV or transdermal narcotic agents for pain control. IV medications allow rapid adjustment to changes in blood pressure and can even be dosed according to blood pressure parameters rather than continuously. Overall, however, surgeon preference is to wean patients off the IV pain medications quickly. Patients are often unable to take oral medications during the early postoperative phase, because abdominal distention and poor bowel sounds are contraindications to beginning oral intake. In addition, oral medications may not be absorbed properly in the face of gastric emptying delay; thus, other routes should be used. (12,25). We found that gastric motility agents were not effective, unless accompanied by pain medication. Thus, we found that transdermal fentanyl was useful to alleviate the gastric distension and manage the autonomic dysreflexia in our patients. It remains unclear whether this medication was itself associated with symptom reduction or whether the problem was already starting to resolve by the time we changed from IV to transdermal narcotics. Fentanyl and morphine have differing affinities for the mu and kappa subtypes of the opioid receptor; thus, the improvement seen with Fentanyl may indeed represent a physiologic difference in the way these medications affect gastrointestinal motility.
It is difficult to know what caused the gastric atony. Possibilities include prolonged intermittent gastric distention (>1 week) or antral hypomotility from the sympathetic output as mentioned above. Despite the beneficial effects of the pain medications discussed above, PEG tube placement was necessary to relieve the gastric distention. Interestingly, both patients recovered gastric function within a week after PEG, for reasons that are not clear, leading us to wonder if they would have gotten better alone or if the decompression played a role in their recovery.
CONCLUSIONS
In summary, this case series documented delayed gastric emptying in 3 persons with tetraplegia after abdominal surgery. In all cases, decreasing narcotic doses seemed to worsen the gastric emptying delays, and increasing narcotic doses reduced the gastric emptying delays. Treating the pain with either IV or transdermal medications seems to be very important in aiding gastric emptying, but the ideal medication and timing of administration are yet to be determined. Hopefully, an increased awareness of this phenomenon postoperatively will prompt earlier recognition and treatment and reduce the number of patients needing PEG or PEJ tubes in the future.
Acknowledgments
The authors acknowledge University of Texas Southwestern Medical Center, Department of Physical Medicine and Rehabilitation, where both authors worked and collaborated on this case series.
REFERENCES
- Gonzales EG, Myer S. Downey and Darling's Physiological Basis of Rehabilitation Medicine. 3rd ed. Butterworth-Heinemann; London: 2001. [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. 10th ed. WB Saunders Co. Ltd; 2000. pp. 697–708. [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. 10th ed. WB Saunders Co. Ltd; 2000. pp. 728–737. [Google Scholar]
- Berne RM, Levy MN, Koeppen BM. Physiology. 5th ed. 2004.
- DeLooze D, Laere M, DeMuynck M, Beke R, Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36:63–66. doi: 10.1038/sj.sc.3100531. [DOI] [PubMed] [Google Scholar]
- Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36:485–490. doi: 10.1038/sj.sc.3100616. [DOI] [PubMed] [Google Scholar]
- Fealey RD, Szurszewski JH, Merritt JL, DiMagno EP. Effect of traumatic spinal cord resection on human upper gastrointestinal motility and gastric emptying. Gastroenterology. 1984;87:69–75. [PubMed] [Google Scholar]
- Telford GL, Go VL, Szurszewski JH. Effect of central sympathectomy on gastric and small intestinal myoelectric activity and plasma motilin concentrations in the dog. Gastroenterology. 1985;89:989–995. doi: 10.1016/0016-5085(85)90198-2. [DOI] [PubMed] [Google Scholar]
- Segal JL, Milne N, Brunnemann SR. Gastric emptying is impaired in patients with spinal cord injury. Am J Gastroenterol. 1990;90:466–470. [PubMed] [Google Scholar]
- Lu CL, Chen JDZ. Gastric myoelectrical activity in patients with cervical spinal cord injury. Am J Gastroenterol. 1998;93:2391–2396. doi: 10.1111/j.1572-0241.1998.00693.x. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Campagnolo D, DeLisa JA. Spinal Cord Medicine. Lippincott, Williams & Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- Kao CH, Ho YJ, Changlai SP, Ding HJ. Gastric emptying in spinal cord injury patients. Dig Dis Sci. 1999;44:1512–1515. doi: 10.1023/a:1026690305537. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chayes Z, Korsten MA, Bauman WA. Gastric emptying rates to liquid or solid meals appear to be unaffected by spinal cord injury. Am J Gastroenterol. 1994;89:1856–1858. [PubMed] [Google Scholar]
- Gondim FAA, Rodrigues CL, Lopes ACA, et al. Effect of preinjury large bowel emptying on the inhibition of upper gastrointestinal motility after spinal cord injury in rats. Dig Dis Sci. 2003;48:1713–1718. doi: 10.1023/a:1025482609323. [DOI] [PubMed] [Google Scholar]
- Enck P, Greving I, Klosterhalfen S, Wietek B. Upper and lower gastrointestinal motor and sensory dysfunction after human spinal cord injury. Prog Brain Res. 2006;152:373–384. doi: 10.1016/S0079-6123(05)52025-9. [DOI] [PubMed] [Google Scholar]
- Read NW, Houghton LA. Physiology of gastric emptying and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 1989;18:359–373. [PubMed] [Google Scholar]
- Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4:365–372. doi: 10.1016/s1470-2045(03)01118-5. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Nasse JS, Rogers RC. Alpha-1 adrenergic input to solitary nucleus neurons: calcium oscillations, excitation and gastric reflex control. J Physiol. 2005;562:553–568. doi: 10.1113/jphysiol.2004.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou-Tan FY, Robertson CS, Chiou GC. Catecholamine assays in a rat model for autonomic dysreflexia. Arch Phys Med Rehabil. 1998;79:402–404. doi: 10.1016/s0003-9993(98)90140-x. [DOI] [PubMed] [Google Scholar]
- Karlsson AK, Fribery P, Lonnroth P, Sullivan L, Elam M. Regional sympathetic function in high spinal cord injury during mental stress and autonomic dysreflexia. Brain. 1998;121:1711–1719. doi: 10.1093/brain/121.9.1711. [DOI] [PubMed] [Google Scholar]
- Leman S, Bernet F, Sequeria H. Autonomic dysreflexia increases plasma adrenaline level in the chronic spinal cord-injured rat. Neurosci Lett. 2000;286:159–162. doi: 10.1016/s0304-3940(00)01111-3. [DOI] [PubMed] [Google Scholar]
- Gao SA, Ambring A, Lambert G, Karlsson AK. Autonomic control of the heart and renal vascular bed during autonomic dysreflexia in high spinal cord injury. Clin Auton Res. 2002;12:457–464. doi: 10.1007/s10286-002-0068-0. [DOI] [PubMed] [Google Scholar]
- Bar-on Z, Ohry A. The acute abdomen in spinal cord injury individuals. Paraplegia. 1995;33:704–706. doi: 10.1038/sc.1995.148. [DOI] [PubMed] [Google Scholar]
- Chen D, Nussbaum SB. The gastrointestional system and bowel management following spinal cord injury. Phys Med Rehabil Clin North Am. 2000;11:45–56. [PubMed] [Google Scholar]
- Mukand JA, Kaplan MS, Blackinton DD, Biener-Bergman S, Trojan DA. The gastric emptying scan as a tool for surgical management of severe bowel dysfunction in spinal cord injury: 2 case reports. Arch Phys Med Rehabil. 2000;81:1531–1534. doi: 10.1053/apmr.2000.7160. [DOI] [PubMed] [Google Scholar]