Abstract
Summary:
Spinal cord injury (SCI) often leads to an impairment of the respiratory system. The more rostral the level of injury, the more likely the injury will affect ventilation. In fact, respiratory insufficiency is the number one cause of mortality and morbidity after SCI. This review highlights the progress that has been made in basic and clinical research, while noting the gaps in our knowledge. Basic research has focused on a hemisection injury model to examine methods aimed at improving respiratory function after SCI, but contusion injury models have also been used. Increasing synaptic plasticity, strengthening spared axonal pathways, and the disinhibition of phrenic motor neurons all result in the activation of a latent respiratory motor pathway that restores function to a previously paralyzed hemidiaphragm in animal models. Human clinical studies have revealed that respiratory function is negatively impacted by SCI. Respiratory muscle training regimens may improve inspiratory function after SCI, but more thorough and carefully designed studies are needed to adequately address this issue. Phrenic nerve and diaphragm pacing are options available to wean patients from standard mechanical ventilation. The techniques aimed at improving respiratory function in humans with SCI have both pros and cons, but having more options available to the clinician allows for more individualized treatment, resulting in better patient care. Despite significant progress in both basic and clinical research, there is still a significant gap in our understanding of the effect of SCI on the respiratory system.
Keywords: Spinal cord injuries, Respiratory function, Respiratory physiology, Mechanical ventilation, Phrenic nerve pacing, Tetraplegia, Paraplegia
INTRODUCTION
Spinal cord injury (SCI) severely compromises both sensory and motor function and affects approximately 11,000 new individuals every year in the United States alone. More than one half of all SCIs (56.4%) occur at the cervical level (1). Cervical SCI often leads to an interruption of the descending bulbospinal respiratory pathways, resulting in respiratory muscle paresis and/or paralysis; the more rostral the level of the injury, the greater the likelihood that a major respiratory impairment will occur. Currently, there are no known cures for muscle paralysis. In cases in which patients are unable to maintain adequate ventilation, long-term mechanical ventilator support is indicated (2,3). Although this treatment is effective, it can also lead to serious medical complications such as infection, pneumonia, atelectasis, and even death (4–7). In fact, the primary cause of death after SCI, regardless of the level of injury, is caused by respiratory insufficiency and complications associated with impaired respiratory function (1,8).
Today, basic research on SCI tends to focus on several areas that target functional restitution and regeneration of the injured neural substrate within the spinal cord. Although this research has generated promising results, there still remain many questions regarding successful regeneration and the complete recovery of motor function (9–11). Basic research on the respiratory system after SCI, however, has focused on ways to use neural pathways that are spared by the injury to restore respiratory function. A recent study that questioned the priorities of spinal cord–injured people stated “… that improvements in respiration and the elimination of ventilator dependence are extremely important to the quality of life, and this topic should be at the forefront of research” (12). Thus, the aim of this review is to describe some of the more recent advances in the field of basic respiratory biology after SCI and to review some of the clinical options that patients have regarding the improvement of ventilatory function after SCI.
NEURAL CIRCUITRY UNDERLYING RESPIRATION
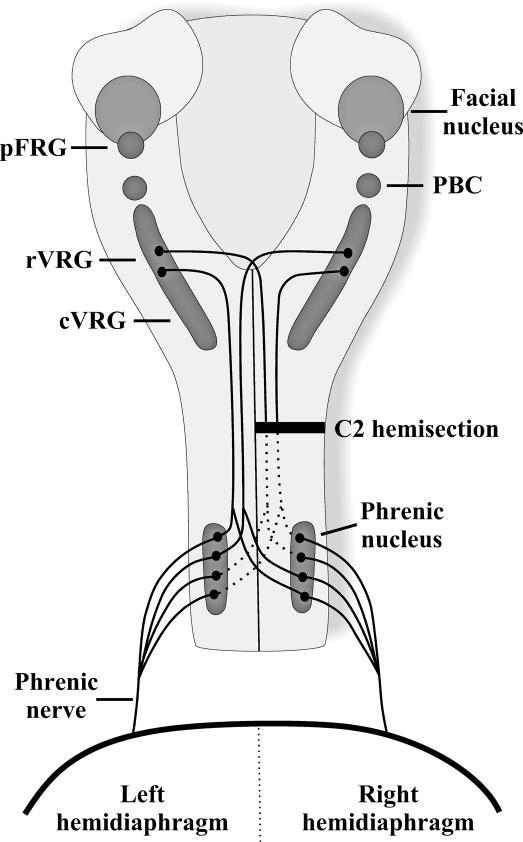
The neural circuitry commanding the activation of the diaphragm has been well described and is based primarily on research that was completed on rats and cats (13,14). The respiratory neural circuitry is made up of neurons located within the brainstem and spinal cord. Respiratory rhythm-generating neurons are located within the pre-Bötzinger complex (PBC) and the para-facial respiratory group (pFRG) situated in the ventral lateral medulla (15,16). These respiratory rhythm-generating neurons are connected to propriobulbar neurons and to premotor neurons located in the ventral respiratory group (VRG), located just ventral and lateral to the nucleus ambiguous (Figure 1). The most rostral part of the VRG (rVRG) contains primarily inspiratory bulbospinal neurons, whereas expiratory bulbospinal neurons are located in the caudal part of the VRG (cVRG). Some of the axons of the bulbospinal VRG neurons cross in the medulla, but others remain uncrossed. These axons descend either unilaterally or bilaterally through the spinal cord in the lateral and ventral funiculi (17–20) and enter the gray matter at the level of the phrenic nucleus where they synapse directly onto phrenic motor neurons (18,21).
Figure 1. Schematic drawing of the major respiratory neural centers and pathways in the rat. Respiratory rhythm generation is formed by neurons located within the pFRG and the PBC. Impulses are transmitted to premotor neurons located within the VRG with inspiratory neurons located predominantly in rVRG and expiratory neurons localized to cVRG. Premotor neurons project either unilaterally or bilaterally down to phrenic motor neurons located on both sides of the spinal cord (C3–C6). Crossed respiratory axons (arising from VRG neurons bilaterally) have been localized at the level of the phrenic nucleus and contribute to the expression of the crossed phrenic phenomenon. Phrenic axons from each side of the spinal cord form the phrenic nerve, which projects to each half of the diaphragm.
The phrenic motor neurons in the ventral horn of the cervical spinal cord extend rostrocaudally as an interrupted column of cells. The specific segmental location of phrenic neurons varies between species: human C3–C5 (22,23), cat C5–C6 (24,25), and rat C3–C6 (26–28). The majority of the dendrites of the phrenic motor neurons run rostrocaudally within the phrenic nucleus; however, some radiate out into the white matter of the spinal cord both laterally and medially. In rats, the primary respiratory projection from the medulla to the phrenic motor nucleus is a bilateral, monosynaptic projection (Figure 1). Upper cervical SCI often disrupts the transmission of the respiratory signal from the brainstem to the phrenic motor neurons, resulting in some paralysis of the diaphragm. Indeed, any injury to the cervical region, even below the phrenic motor nucleus, may affect the transmission of respiratory impulses to the thoracic and abdominal musculature, resulting in muscle paralysis that alters breathing significantly.
Although contusion injuries closely parallel the types of injuries sustained by humans, a hemisection, in which the spinal cord is incised on 1 side of the spinal cord (either left or right) at the second cervical segment, effectively interrupts the descending respiratory projections to the phrenic nucleus ipsilateral to the hemisection, thereby paralyzing the ipsilateral hemidiaphragm. This type of injury produces a precise lesion and allows one to know exactly which respiratory axons are injured and which axons are spared. Thus, the hemisection model has been used in many basic science studies involving respiration after SCI (29).
After a C2-hemisection injury, paralysis of the ipsilateral hemidiaphragm results in a significant reduction in tidal volume and an increase in breathing frequency. This has been shown in unanesthetized (30,31) and anesthetized, spontaneously breathing rats (32). The changes in breathing pattern observed after SCI are the result of vagal feedback, most likely arising from changes in lung and chest wall compliance caused by the paralyzed hemidiaphragm and intercostal muscles. Studies performed on C2-hemisected rats show that, although the breathing pattern is significantly altered in response to SCI, total ventilation (breathing frequency × tidal volume) remains unchanged (31,32).
Recently, contusion models have been used to examine the effect of high SCI on respiration (33–35). Baussart et al (35) developed a C2-contusion injury that displays respiratory patterns similar to a C2-hemisection injury and allows one to examine the activation and neuroplasticity of latent respiratory pathways similar to the hemisection model. The contusion model also allows one to examine various strategies that are unique to the repair of the contused spinal cord. Such strategies as neuroprotection and repair of contused axons, regeneration and reconnection of descending premotor fibers with motor neurons, and methods aimed at targeting the reduction of primary and secondary processes of injury to increase axonal sparing can now be performed in a C2-contusion model using the respiratory motor system to measure improved motor function.
HISTORY OF THE CROSSED PHRENIC PHENOMENON
The respiratory system was designed with redundant motor pathways that contain latent respiratory fibers that can be activated to restore some motor function after SCI. In a series of seminal experiments, Porter (36) showed one of the first examples of motor recovery in the respiratory system after SCI. In rabbits and dogs, Porter paralyzed the ipsilateral hemidiaphragm by making a spinal cord hemisection rostral to the level of the phrenic motor nucleus. Subsequent transection of the contralateral phrenic nerve resulted in complete paralysis of the diaphragm. The significant increase in the drive to breathe activated a latent pathway that restored function to the hemidiaphragm paralyzed by hemisection and was later termed the “crossed phrenic phenomenon” (37). Further examination revealed that the amount of activity recorded from the previously paralyzed hemidiaphragm was proportional to the intensity of the respiratory drive (38). The presence of the crossed phrenic phenomenon has since been confirmed in dogs (37–39), cats (37–41), rabbits (37,41–43), guinea pigs (44,45), rats (35,46–48), mice (49), and even woodchucks (37).
The neural pathway mediating the crossed phrenic phenomenon in rats was revealed using a retrograde, transsynaptic neural tracer (50). The study revealed that the neural substrate underlying the crossed phrenic phenomenon in rats is a monosynaptic connection composed of bulbospinal axons that arise bilaterally in the VRG, project down the spinal cord contralateral to the hemisection, and decussate in the spinal cord to synapse onto phrenic motor neurons (Figure 1). This pathway is referred to as the “crossed phrenic pathway” (CPP) (50). Although it was reported that no phrenic motor neuron dendrites in adult rats crossed the midline of the spinal cord (51), Prakash et al (52) did observe a small amount of spinal decussating phrenic dendrites in adult rats. Therefore, the CPP may involve respiratory impulses that cross the midline over both descending premotor axons, as well as dendrites from the contralateral side that cross in the spinal cord and receive descending inputs (29).
ACTIVATION OF THE CROSSED PHRENIC PATHWAY
Traditionally, the CPP was induced using asphyxia. In fact, either hypoxia (53–55) or hypercapnia (53,54,56) alone is sufficient to illicit the CPP. Studies now typically measure the amount of recovered activity in the phrenic nerve ipsilateral to hemisection, referred to as crossed phrenic activity (57). In general, most methods that enhance phrenic motor output seem capable of converting the ineffective CPP into an effective motor pathway. For example, in rats, administration of theophylline, a respiratory stimulant (58), activated the CPP and restored respiratory function after a C2 hemisection (59). Follow-up experiments indicated that theophylline acted primarily through the adenosine-A1 receptor (60), but the magnitude of the recovery could be further enhanced by targeting both A1 and A2 receptors. Specifically, studies that simultaneously targeted central A1 receptors (DPCPX, a specific A1-receptor antagonist) and peripheral A2 receptor CGS-21680 (a peripherally specific A2-receptor agonist) enhanced the magnitude of recovered function over that attained by A1-receptor blockade alone (61,62).
Interestingly, chronic administration of theophylline (3 times per day for 3 days) not only induced respiratory recovery through the activation of the CPP, but the recovery persisted (60). The drug-induced activation of the CPP was sustained for at least 1 month after treatment; long after theophylline was cleared from blood as determined by blood serum analysis. The mechanism underlying the drug induced plasticity is unknown, but ongoing studies are addressed at resolving this issue.
In most species, crossed phrenic activity can be expressed within minutes after spinal hemisection, but not in guinea pigs and young adult rats (45,46). Studies have shown that morphologic changes occur within the rat phrenic nucleus within hours after hemisection, such as increases in the number of dendrodendritic appositions and the number of excitatory synapses, as well as lengthening of the synaptic active zones (63,64). These changes may improve the efficacy of the communication between VRG neurons and phrenic motor neurons. If sufficient time is allowed after hemisection before cutting the contralateral nerve, 3.5 hours for the guinea pig (45), 0.5 hours for the rat (57), and approximately an hour for the mouse (49), the CPP can be activated. The amount of crossed phrenic activity that can be induced continues to increase as the interoperative interval is lengthened. In fact, over time, crossed phrenic activity appears spontaneously after C2 hemisection (65). Nantwi et al (65) reported that spontaneous crossed phrenic activity begins to appear in female rats 6 weeks after hemisection. By 12 weeks after injury, all female rats showed spontaneous crossed phrenic activity. Other studies have reported that crossed phrenic activity appears at even earlier time-points in male rats, at 2 (31,55) and 4 weeks (31), suggesting that there may be sex differences or possibly differences between individual rat strains.
Although morphologic changes occur after SCI, which seem to enhance motor neuron firing, it is possible that the functionally ineffective synaptic terminal may be physiologically normal. In other words, the synapse is functional and neurotransmitter is released, but the amount of synaptic input over the CPP may be quantitatively insufficient to fully depolarize phrenic motor neurons after SCI (63). In addition, the size of phrenic motor neurons decreases after SCI (66). Because smaller neurons are more excitable because of the Henneman principle (67), it would be more likely that the existing crossed input would activate phrenic motor neurons and cause contraction of the diaphragm.
Synaptic Strengthening Onto Phrenic Motoneurons
Exposure to acute intermittent hypoxia (a series of short-term hypoxic episodes separated by episodes of normoxia) leads to a type of neural plasticity that strengthens synaptic pathways to phrenic motor neurons. This plasticity, referred to as phrenic long-term facilitation (pLTF), is expressed as a gradual increase in phrenic burst output that occurs after the last exposure to hypoxia and lasts for many minutes to hours (68,69). The underlying mechanisms that contribute to the increase in phrenic motor output include serotonin receptor activation and downstream signaling cascades with brain-derived neurotrophic factor (BDNF) (70–72), which lead to the strengthening of excitatory glutamatergic synapses between VRG neurons and phrenic motor neurons, similar to mechanisms underlying long-term potentiation (LTP). Indeed, McGuire et al (73) showed that blocking N-methyl-D-aspartate (NMDA) receptors with MK-801 eliminates pLTF produced by intermittent hypoxia.
Golder and Mitchell (55) found that acute intermittent hypoxia was able to illicit pLTF in rats that had sustained a chronic SCI, 8 weeks after injury, but not in rats in which the injury was more acute, 2 weeks after injury. The lack of pLTF in acutely injured rats was correlated with an injury-induced reduction in serotonin content around phrenic motor neurons. As time progressed after injury, the serotonin content increased, and pLTF was elicited. This was mediated through the serotonin 2A (5HT-2A) receptor because ketanserin, a 5HT-2A receptor antagonist, blocked pLTF (55).
Because SCI alters the breathing pattern such that the output of the contralateral nerve may be increased, Doperalski and Fuller (74) hypothesized that the contra-lateral nerve of C2-hemisected rats might not have as great a capacity for plasticity as the ipsilateral nerve. They examined the effect of intermittent hypoxia on both nerves of chronic injured (4 and 8 weeks) rats. They found that pLTF did not occur in the contralateral nerves at 4 and 8 weeks after injury but that it did occur in the ipsilateral nerves.
Chronic intermittent hypoxia induces pLTF that is also serotonin dependent; however, data have shown that the neural plasticity occurs not only in the spinal cord but also in central respiratory neurons (75,76). Similar to acute intermittent hypoxia, pLTF was only observed on the nerve ipsilateral, but not contralateral, to hemisection (54). Subsequent exposure to hypoxia and hypercapnia showed a greater ventilatory response in chronic intermittent hypoxia exposed rats compared with normoxia, but again, only in the ipsilateral nerve (54), indicating that neural plasticity of the VRG–phrenic pathway is possible after SCI.
Other studies also indicate that serotonin plays a significant role in the activation of the CPP. Examination of serotonin-labeled terminals using electron microscopy, before and after C2 hemisection (30 days), revealed that the number of serotonin immunoreactive terminals contacting phrenic motor neurons was significantly elevated in hemisected rats (77). Moreover, depletion of serotonin significantly attenuated the morphologic changes induced by C2 spinal hemisection (78). Finally, if serotonin content is elevated in rats with an acute injury (1 day after hemisection) using 5-hydroxytryptophan (5-HTP), crossed phrenic activity can be induced (79). These studies indicate that levels of serotonin must be sufficient to activate the CPP.
In addition to the critical role of serotonin, pLTF also requires the activation of NMDA receptors within the phrenic nucleus in normal rats (73). Although no one has shown that glutamate receptors are involved in pLTF after SCI, it has been shown that upregulation of the NR2A subunit of the NMDA receptor results in the activation of the CPP (80). Collectively, the data suggest that NMDA and serotonin receptors play critical roles in the mechanisms underlying neuroplasticity, leading to the activation of the CPP after SCI.
Disinhibition of Phrenic Motor Neurons
Both acute (81) and chronic (47) cervical dorsal root rhizotomy activated the CPP after SCI. In acute C2-hemisected rats, the EMG activity of the previously paralyzed hemidiaphragm progressively increased as each successive dorsal root (C3–C8) was transected (81). After chronic cervical dorsal root rhizotomy, stimulation of the contralateral ventrolateral funiculus led to greater phrenic potentials in C2-hemisected rats compared with C2 hemisected rats without rhizotomy (47). Because cervical dorsal root rhizotomy increases serotonin innervation of phrenic motor neurons in the spinal cord (82), Fuller et al (47) suggested that the increased serotonin may act on 5HT-2A receptors located on phrenic motor neurons to increase their excitability and activate the ineffective crossed phrenic pathways. Another possibility is that the increased serotonin levels may also act in the dorsal horn on 5HT-1A receptors to decrease the excitability of dorsal horn sensory neurons (83). After SCI, dorsal horn sensory neurons become hyperexcitable, and studies have shown that an agonist of the 5HT-1A receptor, 8-hydroxy-DPAT-hydrobromide (8-OH-DPAT), can decrease the excitability of dorsal horn sensory neurons and attenuate mechanical allodynia and thermal hyperalgesia observed after SCI (84).
In the respiratory system, stimulation of cervical dorsal afferents during the inspiratory phase results in the inhibition of phrenic motor output (85). This reflex is a segmental spinal reflex that is most likely mediated through nonrespiratory interneurons located in the dorsal horn of the spinal cord (86,87). Application of 8-OH-DPAT, a 5HT-1A agonist, to the dorsal horn of the spinal cord increased phrenic motor output and activated the CPP (83). Thus, phrenic motor neurons were most likely disinhibited. Blocking the GABAA receptor, not the glycine receptor, resulted in the enhancement of phrenic motor output and the activation of the CPP (13).
CERVICAL SCI INDUCES SUPRASPINAL PLASTICITY
Within the respiratory system, sensory feedback is altered because of the elimination of one half of the spinal cord and through altered vagal receptor feedback originating from the changes in breathing pattern (see above). Golder et al (89) found that chronic SCI also altered supraspinal respiratory motor output. Phrenic neuro-grams of anesthetized, vagotomized, ventilated rats showed that resting phrenic frequency and hypoglossal motor output was significantly decreased bilaterally 2 months after injury, strongly indicating that supraspinal plasticity may also occur as a result of high cervical SCI. Preliminary data (90) using spinal cord–injured neonates and the in vitro brainstem spinal cord preparation support the finding of a reduced central respiratory drive after injury. SCI causes an alteration in neurotransmitters and proteins both below and above the injury (91–93), and preliminary data showed that, after a high cervical SCI, changes in protein levels extend into the brainstem. Changes in protein levels within the brainstem could potentially cause a direct change in the respiratory motor output (90), as well as alter other autonomic control systems located within brainstem regions.
DEVELOPMENT OF THE CROSSED PHRENIC PATHWAY
Numerous studies have shown that neuronal pathways grow extensively during fetal development and that nonfunctional neural projections are eliminated (94,95), even in the respiratory system (96–98). Because the CPP is latent in adult rats, but remains intact, it was hypothesized that this pathway must have been active during development. A study using the in vitro, brainstem–spinal cord preparation showed that the CPP was active, not latent at birth (99). Ongoing studies are aimed at determining the development of the crossed phrenic pathway in vivo.
In neonates, neurons are relatively more depolarized and thus more excitable (100). The hyperexcitability is caused by many factors including differences in ion channel composition and a higher resting membrane potential (13). In addition, phrenic motor neurons contain gap junction proteins early in development (101,102). Thus, in the neonate any crossed phrenic input might be translated into phrenic motor neuron firing, whereas in the adult relatively more crossed phrenic input may be needed to generate an action potential.
The amount of crossed phrenic activity that could be induced was also compared between young (9–10 wk old) and older adult (9–10 months old) female Sprague-Dawley rats (103). In both age groups, crossed phrenic activity was induced 30 minutes after spinal hemisection, but there was almost a fourfold enhancement of crossed phrenic activity generated in older rats. It is not clear what happens to motor neurons and the respiratory neural circuitry as rats mature. Is there a gradual reorganization of the respiratory neural circuitry that enables older animals to more strongly express crossed phrenic activity? What happens to the respiratory neural pathway between early neonatal stages and the adult? At this point, the existing data generate more questions than answers.
FUNCTIONAL SIGNIFICANCE OF THE CROSSED PHRENIC PATHWAY
Weeks to months after SCI, the CPP becomes spontaneously active (65). The extent to which this increased muscle activity translates into a functional increase in respiratory capacity is not clear. Two recent studies have addressed this question (31,53). The first study (53) showed that spontaneous expression of the CPP did contribute functionally to respiration after SCI. Two months after SCI, crossed phrenic activity was spontaneously present in C2 hemisected rats; however, in rats with a hemisection plus phrenicotomy (dual injury), no crossed phrenic activity was present. Under anesthetized, spontaneously breathing conditions, the rapid, shallow breathing patterns were not different between the 2 groups, indicating that, under normal “resting” conditions, the CPP did not contribute functionally to respiratory capacity. However, under conditions when respiratory capacity was elevated, the rats with the dual injury were unable to generate as large inspiratory volumes as the hemisected-only rats. During hypercapnia, hypoxia, and asphyxia, the contralateral phrenic output in the dual-injured group was larger than the hemisected-only rats, indicating that dual-injured rats needed a larger contralateral output to increase ventilation sufficiently. The data suggest that the C2-hemisected rats were able to use the spared CPP to generate larger inspiratory volumes when necessary. Thus, the CPP contributed significantly to respiratory function during conditions of elevated respiratory drive. The second study found that the presence of spontaneous crossed phrenic activity did not contribute functionally to respiration under normal conditions or during hypercapnia (31).
The 2 studies clearly show that under normal conditions, the amount of crossed phrenic activity expressed spontaneously in chronic spinal cord–injured rats is not contributing to an increase in tidal volume. Why 1 study showed that crossed phrenic activity may contribute to increased ventilation during hypercapnia and 1 did not may simply reflect a difference in the time after hemisection, the rat strain used and the amount of crossed phrenic activity expressed. Recall that Nantwi et al (65) did not detect any spontaneous crossed phrenic activity in female rats until 6 weeks after hemisection. The recovery was not significantly enhanced until 12 weeks after hemisection. Further studies are needed to adequately address whether the spontaneous expression of crossed phrenic activity contributes toward a functional role in respiration.
TREATMENT OF RESPIRATORY INSUFFICIENCY IN HUMANS WITH SCI
Respiratory insufficiency is reported as the number one cause of morbidity and mortality in patients who survive a SCI (1,8). Studies consistently report that SCI negatively impacts respiratory function in humans (1,8,104–106). Not surprisingly, the amount of respiratory impairment is relative to the level of the lesion. Many patients who sustain a cervical SCI will require some form of mechanical ventilatory support (3–5,8). During the first year after injury, however, improvements in respiratory function occur spontaneously, and only 5% of patients require further ventilatory support. Even in lower level tetraplegia and paraplegia, improvements in lung function are observed over the first year (106). This is not unusual because small improvements in general motor function usually occur during the first year after injury (107,108). After the first year, however, very little improvement is noted; sometimes a loss of function is even observed (104).
The CPP has not been shown in humans. However, all mammals tested so far (mice, rats, guinea pigs, rabbits, cats, dogs, woodchucks) have shown that the CPP is present. Because mammals are the only class of vertebrates that possess a diaphragm, the CPP is most likely a mammalian feature and present in humans. Demonstration of the CPP in humans is difficult. Often, SCI is so severe that both the right and left sides are affected, masking the presence (or absence) of any crossed pathways. In addition, there have been no studies designed to examine whether the CPP exists or can be activated in humans. One case study (109), however, did indicate that the CPP may exist in humans. In a patient with chronic asymmetric C5–C7 tetraplegia and respiratory insufficiency, theophylline increased diaphragm function and showed a greater increase in function on the side with the more rostral injury, strongly indicating that the CPP may have been activated to restore function to the previously impaired muscle.
In the respiratory system, forced vital capacity (FVC), forced expired volume in 1 second (FEV1), and peak expiratory flow rates (PEFR) are normal in people with low-level paraplegia that never smoked (104). However, these respiratory parameters are all reduced in smokers and in people with a more rostral level of paraplegia and tetraplegia (104). Maximum inspiratory pressures (MIP) and maximum expiratory pressures (MEP) are also significantly reduced in patients with tetraplegia (106). Moreover, the loss of muscle tone and respiratory function leads to a high incidence of sleep disordered breathing that occurs within 4 weeks after cervical SCI (106). Most importantly, however, a greater loss of respiratory function was observed in patients with a longer duration of injury, independent of age in both paraplegia (105) and tetraplegia (104). The research on humans clearly indicates that efforts should be made to help patients increase ventilatory function and minimize respiratory complications after SCI.
Apart from drugs given to increase respiratory function immediately after injury, several methods have been used to restore respiratory motor function (primarily inspiratory) after SCI in humans (110,111). These include muscle training regimens that target specific respiratory muscles to improve ventilatory function (112), and electrical and magnetic stimulation techniques aimed at the activation of the diaphragm and intercostal muscles to allow patients to safely wean from ventilators (113).
Respiratory Muscle Training
Van Houtte et al (112) and Brooks et al (114) attempted to perform a meta-analysis of the research to evaluate the effectiveness of respiratory muscle training in patients with SCI on respiratory muscle strength, endurance, and pulmonary function. However, the data retrieved from the literature were insufficient to perform an adequate meta-analysis. This underscores the need for more careful, controlled studies examining respiratory muscle training regimens after SCI. The resultant systematic review of the literature (112), however, did reveal that respiratory muscle training regimens tended to improve expiratory muscle strength, vital capacity, and residual volume after SCI. Even other forms of muscle training regimens, such as wheelchair interval training (115) or providing trunk and abdominal support (116), have reported improvements in ventilation or the efficiency of ventilation. Studies indicate that exercise regimens are extremely important in the improvement of other motor functions after SCI (117,118). In a recent questionnaire that asked patients with spinal cord injury what they thought would improve their quality of life, an overwhelming majority indicated that “exercise was important to functional recovery” (12). However, more than one half indicated that they did not have access to exercise or a trained therapist to oversee the exercise. Another major limitation, as well, is that even if a muscle training regimen is started, most studies reveal that patients discontinue the exercise within months unless some major motivational or monitoring strategy has been used (119).
Numerous animal studies indicate that exercise may improve motor function by a form of neural plasticity induced through serotonin, BDNF, and glutamate receptor signaling in the spinal cord (120,121). Thus, it is easy to speculate that because serotonin, BDNF, and NMDA receptors are involved in forms of long-term neural plasticity of the respiratory system (eg, pLTF) and serotonin, BDNF, and NMDA receptors are involved in exercise-induced neural plasticity; serotonin, BDNF, and NMDA receptors may also be an underlying mechanism behind improvements in respiratory motor function after respiratory muscle training programs.
Phrenic Nerve Pacing, Diaphragm Pacing, and Combined Intercostal and Diaphragm Pacing Techniques
Diaphragm muscle pacing, phrenic nerve pacing, and combined intercostal and unilateral diaphragm pacing techniques are currently being used to wean patients from ventilators and reduce the incidence of infection, atelectasis, and respiratory failure (113). Phrenic nerve pacing is a clinically accepted method that entails a thoracotomy to implant electrodes on the phrenic nerve (122). It gives patients improved speech, increased comfort, and mobility along with a reduction in health care costs compared with mechanical ventilation. However, phrenic nerve pacing does have a relatively high risk of nerve damage or deterioration associated with its placement. Diaphragm pacing, on the other hand, requires a less invasive procedure that does not require long hospitalization after implantation, but it costs more initially. Currently, diaphragm pacing protocols have electrode wires exiting the skin that carry a small risk of infection. However, an implantable system similar to that used with conventional phrenic nerve pacing is under development (113). Clinical trials with both the phrenic nerve pacing and diaphragm pacing methods showed that, after a period of reconditioning, which is required to increase muscle tone because of muscle atrophy, lung volume is significantly improved, and most patients gain full independence and no longer require ventilator assistance (113).
Techniques targeting the upper intercostal muscles alone have been less successful. Patients who had injured phrenic nerves and were not good candidates for either diaphragm or phrenic nerve pacing were able to participate in a clinical trial to examine whether inspiratory intercostal muscle pacing (electrodes placed on T2–T3) would increase respiratory function (123). Stimulation only resulted in modest increases in inspired volumes, and patients were unable to wean completely from the ventilator; patients could only breathe unaided from 20 minutes to 2 to 3 hours. Combining intercostal and diaphragm pacing, however, might be a feasible option when 1 side of the diaphragm is unable to support diaphragm pacing. This approach has shown promising results, and clinical trials indicate that patients can be maintained for 16 to 24 h/d without ventilator support (123).
Others, however, argue that high costs and potential complications of surgery indicate that alternative methods of ventilatory support other than traditional mechanical ventilation and phrenic nerve pacing should be considered for the patient with SCI (124,125). Noninvasive mechanical ventilation and body ventilators have been suggested as alternatives that avoid intubation and its associated risks; thus far, this procedure is only based on case reports or case series, and no clinical studies have evaluated the effectiveness of this procedure in patients with SCI (126). Noninvasive intermittent positive airway pressure can be administered through the mouth, nose, or custom strapless oral–nasal interface, and it has been used successfully to treat patients with high level tetraplegia who were unable to sustain ventilation on their own (125–128). The lack of an endotracheal tube affords these patients the ability to vocalize and eat orally and has a much lower risk of respiratory infections than standard mechanical ventilator use. Noninvasive mechanical ventilation, however, is not without its associated problems such as ischemia and necrosis at the skin–mask interface, gastric distention from high pressures, mucosal drying and thickening, and possible emesis and aspiration (111,129). Although each method of ventilatory aid has its pros and cons, having multiple options available to the clinician allows for more individualized treatment, resulting in better patient care.
Thus far, the options discussed that are currently available to the patient with SCI, respiratory muscle training, phrenic pacing, diaphragm pacing, or even intercostal muscle pacing, target the inspiratory muscles only. The expiratory muscles are also extremely important for normal respiratory function. Some patients have normal spirometry values; however, they lack the abdominal strength to generate adequate cough. Coughing clears the lungs of foreign material and greatly reduces the amount of lung infections. Currently, studies are underway to restore expiratory muscle function to restore effective cough mechanisms (130,131). Initial studies showed that surface electrodes were not sufficient in increasing abdominal and lower thoracic pressures. Animal studies, however, showed that electrodes implanted in the lower spinal cord at T9 and T13 can activate intercostal and abdominal muscles to generate sufficient pressures that could aid in coughing mechanisms (132). Restoration of the cough may greatly reduce the risk of respiratory infection and prevent atelectasis and respiratory failure in patients with SCI.
Drug Administration
Because animal studies indicated that theophylline was capable of improving respiratory function after SCI, and theophylline was already an Food and Drug Administration (FDA)-approved drug used for respiratory insufficiency (infant apneas, asthma, and chronic obstructive pulmonary disease), the effect of theophylline was examined in patients with SCI and a history of respiratory insufficiency (109,133,134). Two case studies showed that theophylline increased respiratory motor output after SCI (109,133). Theophylline resulted in an increase in central respiratory drive and inspiratory muscle force in 1 patient with chronic asymmetric C5–C7 tetraplegia and a history of respiratory insufficiency (109). Another patient with C5–C6 chronic tetraplegia was quickly and effectively weaned from a ventilator with theophylline (133). The patient had contracted pneumonia and septicemia years after the SCI, developed respiratory failure, and was placed on a ventilator. After resolution of the primary infections, the patient was unable to wean from the ventilator because of respiratory insufficiency, but within 1 day of theophylline treatment, the patient successfully weaned from the ventilator (133). The extremely positive results from these 2 case studies led to the design of a double-blind, placebo-controlled crossover study to examine the effect of theophylline on respiratory muscle strength in 10 patients with chronic tetraplegia (134). Although the mean data were not significant, 40% of the patients did show some positive changes in response to theophylline. The lack of significant group differences may have been because of the small sample size or that the study did not recruit patients with a history of respiratory insufficiency. Indeed 24 patients were initially recruited, but the drug was not well tolerated by most, and only 10 patients completed the study. More thorough studies are needed to fully address the potential therapeutic effects of theophylline in spinal cord–injured patients.
Some studies indicated that muscle spasticity may also affect respiration after SCI (135,136). For example, in a recent case study, baclofen was administered to a C4-level patient who was unable to wean from the ventilator (136). After baclofen treatment and subsequent reduction in muscle spasticity, the patient quickly and successfully weaned from the ventilator. The authors concluded that clinical trials should address whether muscle spasticity interferes with respiratory function and determine whether reducing spasticity results in improved respiratory function in patients with SCI. Animal studies involving a combination of baclofen and theophylline therapy to improve respiratory function are currently underway in our laboratory.
SUMMARY
SCI at any level can lead to an impairment of the respiratory system. The long-term goal of basic and clinical research is to effectively treat patients with SCI, improve the quality of life, and significantly decrease mortality rates associated with respiratory insufficiency. Researchers now have contusion and hemisection animal models available to examine repair, restoration, and synaptic plasticity strategies to improve respiratory function after injury. The recent availability of the mouse as a SCI model of respiratory insufficiency now allows the use of genetic manipulation and the possible discovery of new genes that may also be involved in those repair, restoration, and synaptic plasticity strategies. Currently, basic research has tended to focus on how spared respiratory neural pathways can be activated to restore function to previously paralyzed muscles by (a) increasing the descending excitatory respiratory drive, (b) strengthening synaptic transmission between bulbospinal descending pathways and phrenic motor neurons, (c) decreasing the firing threshold level in phrenic motor neurons, and (d) decreasing the inhibitory inputs on phrenic motor neurons. Data suggest that some if not all of these approaches may also be implemented in the chronically injured animal, showing possible mechanisms for future clinical studies.
Human studies suggest that respiratory muscle training regimens may improve respiratory function; however, it seems that few studies have carefully addressed this issue. Patients with SCI realize the importance of exercise, yet very few have access to trained therapists to oversee exercise programs. Because the primary cause of death is attributed to respiratory insufficiency in all patients, research needs to be targeted at improving respiratory function in all patients with SCI, not just those currently experiencing respiratory insufficiency. The data from both the basic and the clinical sciences have shown considerable progress over recent years in our understanding of how SCI alters respiratory function. However, it is clear that more research is needed to address all of the concerns surrounding the control of respiratory function after SCI.
Acknowledgments
This work was funded by NIH Grants HD-35766 (K. Nantwi) and HD-31550 (H. G. Goshgarian).
REFERENCES
- National Spinal Cord Injury Statistical Center Spinal cord injury: facts and figures at a glance. J Spinal Cord Med. 2006;29:89–90. [Google Scholar]
- Epstein SK, Nevins ML. Prolonged mechanical ventilation. In: Mosenfifar Z, Soo Hoo GW, editors. Practical Pulmonary and Critical Care Medicine: Respiratory Failure. Lung Health in Biology and Disease. New York: Taylor and Francis Group; 2006. pp. 187–217. [Google Scholar]
- Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Claxton AR, Wong DT, Chung F, Fehlings MG. Predictors of hospital mortality and mechanical ventilation in patients with cervical spinal cord injury. Can J Anaesth. 1998;45:144–149. doi: 10.1007/BF03013253. [DOI] [PubMed] [Google Scholar]
- Fishburn MJ, Marino RJ, Ditunno JF., Jr. Atelectasis and pneumonia in acute spinal cord injury. Arch Phys Med Rehabil. 1990;71:197–200. [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, et al. Long term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Pugin J, Oudin S. Cellular and molecular basis for ventilator-induced lung injury. In: Dreyfuss D, Saumon G, Hubmayr RD, editors. Lung Biology in Health and Disease: Ventilatory-induced Lung Injury. New York: Taylor and Francis Group; 2006. pp. 205–222. [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Dietz V, Curt A. Neurological aspects of spinal cord repair: promises and challenges. Lancet Neurol. 2006;5:688–694. doi: 10.1016/S1474-4422(06)70522-1. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S. Regeneration following spinal cord injury, from experimental models to humans: where are we? Expert Opin Ther Targets. 2006;10:363–376. doi: 10.1517/14728222.10.3.363. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal crod of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath: prenatal maturation of respiratory neural control. J Physiol. 2006;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem-bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron-microscopic evidence for monosynaptic connections. J Comp Neurol. 1990;302:707–714. doi: 10.1002/cne.903020403. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motorneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenburger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- Routal RV, Pal GP. Location of the phrenic nucleus in the human spinal cord. J Anat. 1999;195:617–621. doi: 10.1046/j.1469-7580.1999.19540617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani NH, Hollinshead WH. Localisation of the phrenic nucleus in the spinal cord of man. Anat Rec. 1956;125:683–699. doi: 10.1002/ar.1091250403. [DOI] [PubMed] [Google Scholar]
- Webber CL, Jr, Wurster RD, Chung JM. Cat phrenic nucleus architecture as revealed by horseradish peroxidase mapping. Exp Brain Res. 1979;35:395–406. doi: 10.1007/BF00236759. [DOI] [PubMed] [Google Scholar]
- Duron B, Marlot D, Larnicol N, Jung-Caillol MC, Macron JM. Somatotopy in the phrenic motor nucleus of the cat as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett. 1979;14:159–163. doi: 10.1016/0304-3940(79)96141-x. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Devries KL, Goshgarian HG. Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol. 1989;104:88–90. doi: 10.1016/0014-4886(89)90013-7. [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Chou SM. Localization of the phrenic nucleus in the rat: a HRP study. Neurosci Lett. 1980;16:119–124. doi: 10.1016/0304-3940(80)90330-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH and respiratory rate in the adult rat. Exp Neurol. 1986;93:440–445. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport DW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998;150:143–152. doi: 10.1006/exnr.1997.6757. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25:4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Plentes J, Tadié M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbleuth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- Lewis LJ, Brookhart JM. Significance of the crossed phrenic phenomenon. Am J Physiol. 1951;166:241–254. doi: 10.1152/ajplegacy.1951.166.2.241. [DOI] [PubMed] [Google Scholar]
- Deason J, Robb LJ. On the pathways for the bulbar respiratory impulses in the spinal cord. Am J Physiol. 1911;28:57–63. [Google Scholar]
- Rosenbaum H, Renshaw B. Descending respiratory pathways in the cervical spinal cord. Am J Physiol. 1949;157:468–476. doi: 10.1152/ajplegacy.1949.157.3.468. [DOI] [PubMed] [Google Scholar]
- Seligman AM, Davis WA. The effects of some drugs on the crossed phrenic phenomenon. Am J Physiol. 1941;143:102–106. [Google Scholar]
- Chatfield PO, Mead S. Role of the vagi in the crossed phrenic phenomenon. Am J Physiol. 1948;54:417–422. doi: 10.1152/ajplegacy.1948.154.3.417. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Klopps CT, Simeone FA. A further study of the crossed phrenic phenomenon. J Neurophysiol. 1938;1:508–520. [Google Scholar]
- Guth L. Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol. 1976;51:414–420. doi: 10.1016/0014-4886(76)90265-x. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Guth L. Demonstration of functionally ineffective synapses in the guinea pig spinal cord. Exp Neurol. 1977;57:613–621. doi: 10.1016/0014-4886(77)90093-0. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol. 1979;66:547–555. doi: 10.1016/0014-4886(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett. 2002;323:25–28. doi: 10.1016/s0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse—the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Furicchia JV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol. 1987;96:621–627. doi: 10.1016/0014-4886(87)90224-x. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23:2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Castro-Moure F, Goshgarian HG. Activation of a latent respiratory motor pathway by stimulation of neurons in the medullary chemoreceptor area of the rat. Exp Neurol. 2001;17:176–184. doi: 10.1006/exnr.2001.7740. [DOI] [PubMed] [Google Scholar]
- O'Hara TE, Goshgarian HG. Quantitative assessment of phrenic nerve functional phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Pena F, Garcia O. Breathing generation and potential pharmacotherapeutic approaches to central respiratory disorders. Curr Med Chem. 2006;13:2681–2693. doi: 10.2174/092986706778201602. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res. 1998;789:126–129. doi: 10.1016/s0006-8993(98)00024-9. [DOI] [PubMed] [Google Scholar]
- James E, Nantwi KD. Involvement of peripheral adenosine A2 receptors in adenosine A1 receptor-mediated recovery of respiratory motor function after upper cervical spinal cord hemisection. J Spinal Cord Med. 2006;29:57–66. doi: 10.1080/10790268.2006.11753857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29:915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Yu XJ, Rafols JA. Neuronal and glial changes in the rat phrenic nucleus occurring within hours after spinal cord injury. J Comp Neurol. 1989;284:519–533. doi: 10.1002/cne.902840404. [DOI] [PubMed] [Google Scholar]
- Sperry MA, Goshgarian HG. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp Neurol. 1993;120:233–244. doi: 10.1006/exnr.1993.1058. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Gosh-garian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- Mantilla CB, Sieck GC. Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol. 2005;567.2:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, et al. Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Tai QT, Palazzolo KL, Goshgarian HG. Synaptic plasticity of 5-hydroxytryptamine-immunoreactive terminals in the phrenic nucleus following spinal cord injury: a quantitative electron microscopic analysis. J Comp Neurol. 1997;386:613–624. [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Willis WD, Hulsebosch CE. Serotonin receptors 5HT1A and 5HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp Brain Res. 2003;49:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- Speck DF, Revelette WR. Attenuation of phrenic motor discharge by phrenic nerve afferents. J Appl Physiol. 1987;62:941–945. doi: 10.1152/jappl.1987.62.3.941. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4-C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res. 1993;93:307–311. doi: 10.1007/BF00228399. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic moto-neurones in cats. J Physiol. 1996;497.3:803–812. doi: 10.1113/jphysiol.1996.sp021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. GABA, not glycine, mediates inhibition of latent respiratory motor pathways after spinal cord injury. Exp Neurol. 2007;200:250–255. doi: 10.1016/j.expneurol.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal cord injury in neonate rats alters respiratory neural output via supraspinal mechanisms. FASEB J. 2004;4:A783–A783. doi: 10.1016/j.expneurol.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–870. [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Alteration in AMPA receptor subunit expression after experimental spinal cord contusion injury. J Neurosci. 1999;19:5711–5720. doi: 10.1523/JNEUROSCI.19-14-05711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75:174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Busetto G, Bidoia C, Favero M, Cangiano A. Activity-dependent synaptic competition at mammalian neuromuscular junctions. News Physiol Sci. 2004;19:85–91. doi: 10.1152/nips.01464.2003. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Cameron WE, He F, Kalipatnapu P, Jodkowski JS, Guthrie RD. Morphometric analysis of phrenic motoneurons in the cat during postnatal development. J Comp Neurol. 1991;314:763–776. doi: 10.1002/cne.903140409. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Developmental aspects of diaphragm muscle cells: structural and functional organization. In: Haddad GG, Farber JP, editors. Developmental Neurobiology of Breathing. New York: Dekker; 1991. pp. 375–428. [Google Scholar]
- Redfern P. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spontaneous crossed phrenic activity in the neonatal respiratory network. Exp Neurol. 2005;194:530–540. doi: 10.1016/j.expneurol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Núñez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons in the cat during postnatal development. Brain Res Bull. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- Reckling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBotzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Halat TJ, El-Maghrabi R, O'Neal MH., III Different expression of connexin 26 and connexin 32 in the pre-Botzinger complex of neonatal and adult rat. J Comp Neurobiol. 2001;440:12–19. doi: 10.1002/cne.1366. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Goshgarian HG. Aging enhances synaptic efficacy in a latent motor pathway following spinal cord hemisection in adult rats. Exp Neurol. 1993;121:231–238. doi: 10.1006/exnr.1993.1090. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Linn WS, Spungen AM, Gong H, Adkins RH, Bauman WA, Waters RL. Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord. 2001;39:263–268. doi: 10.1038/sj.sc.3101155. [DOI] [PubMed] [Google Scholar]
- Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil. 2005;86:1193–1199. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Yilmaz F, Sahin F, Aktug S, Kuran B, Yilmaz A. Long term follow-up of patients with spinal cord injury. Neurorehabil Neural Repair. 2005;19:332–337. doi: 10.1177/1545968305280210. [DOI] [PubMed] [Google Scholar]
- Sipski ML, Jackson AB, Gomez-Martin O, Estores I, Stein A. Effects of gender on neurologic and functional recovery after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1826–1836. doi: 10.1016/j.apmr.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Ferguson GT, Khanchandani N, Lattin CD, Goshgarian HG. Clinical effects of theophylline on inspiratory muscle drive in tetraplegia. Neurorehabil Neural Repair. 1999;13:191–197. [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. [PMC free article] [PubMed] [Google Scholar]
- Consortium for Spinal Cord Medicine Respiratory management following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2005;28:259–293. doi: 10.1080/10790268.2005.11753821. [DOI] [PubMed] [Google Scholar]
- Van Houtte S, Vanlandewijck Y, Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir Med. 2006;100:1886–1895. doi: 10.1016/j.rmed.2006.02.029. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Restoration of respiratory muscle function following spinal cord injury: review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147:273–287. doi: 10.1016/j.resp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Brooks D, O'Brien K, Geddes EL, Crowe J, Reid WD. Is inspiratory muscle training effective for individuals with cervical spinal cord injury? A qualitative systematic review. Clin Rehabil. 2005;19:237–246. doi: 10.1191/0269215505cr856oa. [DOI] [PubMed] [Google Scholar]
- Le Foll-de Moro D, Tordi N, Lonsdorfer E, Lonsdorfer J. Ventilation efficiency and pulmonary function after a wheelchair interval-training program in subjects with recent spinal cord injury. Arch Phys Med Rehabil. 2005;86:1582–1586. doi: 10.1016/j.apmr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Hart N, Laffont I, de la Sota AP, et al. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Arch Phys Med Rehabil. 2005;86:1447–1451. doi: 10.1016/j.apmr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86:1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- King AC, Taylor CB, Haskell WL, Debusk RF. Strategies for increasing early adherence to and long-term maintenance of home-based exercise training in healthy middle-aged men and women. Am J Cardiol. 1988;61:628–632. doi: 10.1016/0002-9149(88)90778-3. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, Van Praag H, Wodktke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antide-pressant. Pharm Biochem Behav. 2003;75:81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Hageman JH, Mauro A, Eisenberg L, Flanigan S, Harvard M. Electrical stimulation of excitable tissue by radio-frequency transmission. Ann Surg. 1964;160:338–350. [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Takaoka Y, Kowalski KE. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil. 2005;86:1200–1207. doi: 10.1016/j.apmr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Bach JR. New approaches in the rehabilitation of the traumatic high level quadriplegic. Am J Phys Med Rehabil. 1991;70:13–19. doi: 10.1097/00002060-199102000-00004. [DOI] [PubMed] [Google Scholar]
- Bach JR, Alba AS. Noninvasive options for ventilatory support of the traumatic high level quadriplegic patient. Chest. 1990;98:613–619. doi: 10.1378/chest.98.3.613. [DOI] [PubMed] [Google Scholar]
- Bach JR. Update and perspectives on noninvasive respiratory muscle aids. Part 1: the inspiratory aids. Chest. 1994;105:1230–1240. doi: 10.1378/chest.105.4.1230. [DOI] [PubMed] [Google Scholar]
- Bach JR, Alba AS, Saporito LR. Intermittent positive pressure ventilation via the mouth as an alternative to tracheostomy for 257 ventilator users. Chest. 1993;103:174–182. doi: 10.1378/chest.103.1.174. [DOI] [PubMed] [Google Scholar]
- Bach JR, Hunt D, Horton JA. Traumatic tetraplegia: noninvasive management in the acute setting. Am J Phys Med Rehabil. 2002;81:792–797. doi: 10.1097/01.PHM.0000027205.42338.72. [DOI] [PubMed] [Google Scholar]
- Soo Hoo GW. Non-invasive ventilation in critical care. In: Mosenifar Z, Soo Hoo GW, editors. Lung Biology in Health and Disease: Practical Pulmonary and Critical Care Medicine: Respiratory Failure. New York: Taylor and Francis Group; 2006. pp. 33–75. [Google Scholar]
- Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest. 1993;103:166–169. doi: 10.1378/chest.103.1.166. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce an effective cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006;173:1386–1389. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 2002;92:2341–2346. doi: 10.1152/japplphysiol.01231.2001. [DOI] [PubMed] [Google Scholar]
- Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127:658–661. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]
- Tzelepis GE, Bascom AT, Badr MS, Goshgarian HG. Effects of theophylline on pulmonary function in patients with traumatic tetraplegia. J Spinal Cord Med. 2006;29:227–233. doi: 10.1080/10790268.2006.11753878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton D, Goldstein B, Jones-Redmond J, Esselman P. Baclofen pump intervention for spasticity affecting pulmonary function. J Spinal Cord Med. 2005;28:343–347. doi: 10.1080/10790268.2005.11753832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont I, Durand MC, Rech C, et al. Breathlessness associated with abdominal spastic contraction in a patient with C4 tetraplegia: a case report. Arch Phys Med Rehabil. 2003;84:906–908. doi: 10.1016/s0003-9993(02)04898-0. [DOI] [PubMed] [Google Scholar]