Abstract
Background/Objective:
Transforaminal epidural steroid injection is a widely utilized nonsurgical strategy for the management of cervical radicular and axial pain. The technique has been shown to be efficacious in relieving the patients' symptoms. Although effective, there are a range of possible complications associated with this procedure. We report the case of a patient with an acute infarction of the cervical spinal cord after a multilevel transforaminal epidural steroid injection.
Methods:
We performed a retrospective chart review of a single case.
Results:
The patient suffered an acute brainstem and cervical spinal cord infarction despite the use of many techniques to minimize the occurrence of vascular injury during the procedure. The patient regained some function after medical and physical therapy.
Conclusions:
This complication, to our knowledge, has only been reported in the literature on 2 other occasions and serves as a reminder of the potentially devastating consequences of performing procedures in proximity to the nervous system.
Keywords: Spinal cord injuries; Myelopathy, vascular; Spinal cord, cervical, infarction, acute; Epidural injection; Methylprednisolone acetate; Dexamethasone
INTRODUCTION
Background
Transforaminal epidural injection of corticosteroids is a widely utilized adjunct in the nonsurgical management of patients with radicular pain, with or without an axial component, originating from the cervical spine (1,2). It is hypothesized that the therapeutic benefit occurs as the result of the suppression of the inflammatory response surrounding the targeted nerve roots (3). The reported incidence of complications in the peri-procedural period is small, and complications include dural puncture, trauma to the spinal nerve, infection, side effects of radiation exposure, vasovagal reaction, and allergic and anaphylactic reaction to the medication (4). Few reports exist on the more devastating complication of spinal cord vascular injury resulting in infarction and neurological deficit.
Case Summary
This 72-year-old, right-handed white woman was transferred to our institution from an outside hospital 2 days after the acute onset of upper extremity paresis and lower extremity plegia after a cervical transforaminal epidural steroid injection for the management of left arm radiculopathy and neck pain. She had a long-standing history of axial cervical spine and radicular pain, having undergone a C4-C5 and C5-C6 anterior cervical discectomy and fusion 3 years prior. Despite initial benefit from surgery, the patient's symptoms returned. Because of multiple comorbid medical conditions, she was managed nonsurgically; treatment included 2 recent uncomplicated cervical transforaminal epidural steroid injections, which provided modest clinical benefit.
Because of the persistence of the patient's symptoms, she received a left-sided cervical transforaminal epidural steroid injection at the C5-C6 and C6-C7 levels. The procedure was performed using mild intravenous midazolam, fentanyl, and propofol sedation; fluoroscopy was utilized to guide the 25-gauge 3.5-inch needle into the foramina. Isovue contrast was injected using magnified live fluoroscopy; after confirmation of contrast around the nerve root sleeve and epidural space and the absence of vascular uptake, 40 mg of methylprednisolone acetate and 0.7 mL of 0.5% bupivacaine were injected in each level. There was no intra- or periprocedural occurrence of hypotension, and the patient transferred from the procedure table to the transport cart without assistance. Approximately 30 minutes postprocedure, the patient complained of lower-extremity weakness and, on examination, was found to be paretic in the upper extremities (left more than right). She was plegic in the lower extremities with some preservation of sensory function, resulting in an American Spinal Injury Association Impairment Scale grade B classification (ASIA-B). High-dose dexamethasone was administered, and an emergency medical resonance imaging (MRI) examination of the cervical spine was obtained. This study did not reveal an extrinsic lesion compressing the spinal cord nor any intrinsic spinal cord parenchymal signal abnormalities. The patient was admitted to the intensive care unit and supported medically; her condition improved over the subsequent 24 hours. Follow-up MRI demonstrated patchy T2 signals within the cervical spinal cord with associated spinal cord expansion, indicative of spinal cord edema secondary to infarction (Figure 1). At that time, the patient was transferred to the neurological surgery service at Northwestern Memorial Hospital for further evaluation and management.
Figure 1. Follow-up MRI showing patchy T2 signals and spinal cord expansion, indicative of spinal cord edema secondary to infarction.
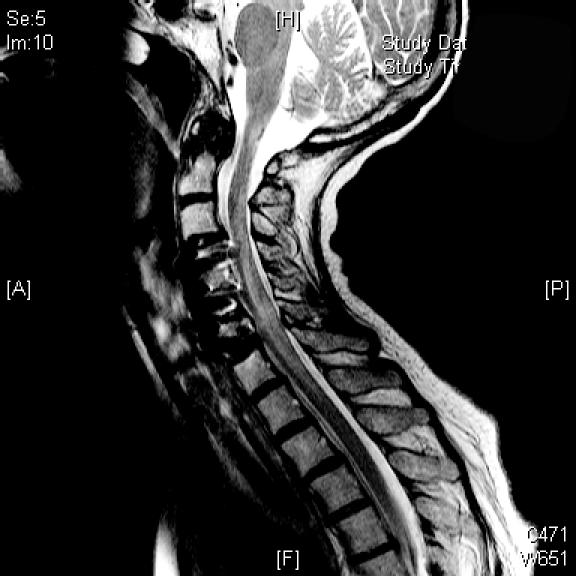
Upon arrival at our institution, the patient underwent an unremarkable cranial nerve examination and had intact diaphragm function but had an incomplete spinal cord injury, including asymmetric strength of upper and lower extremities (right greater than left), and a T4 sensory level (ASIA-C). Given the patient's extensive cardiovascular comorbidities, which included myocardial infarction and a history of coronary artery disease requiring stenting, and an improving neurological condition, she was managed using hemodynamic support with maintenance of the mean arterial blood pressure greater than 85 mmHg via volume resuscitation and without intravenous vasoactive agents. The high-dose dexamethasone, which was initiated within 30 minutes of symptom onset and continued until her transfer, was discontinued. Cervical spine MRI examination at our institution corroborated the outside MRI procedure; in addition, MRI examination of the brain demonstrated restricted diffusion in the medulla, confirming the clinical suspicion of acute infarction involving the cervical spine and extending to the cervicomedullary junction. Physical and occupational therapy did not result in further short-term improvement of the patient's motor or sensory functioning, and the patient was transferred to a rehabilitation facility.
DISCUSSION
It is widely recognized that nonsurgical strategies can result in improved symptom control among patients with radiculopathy and neck pain, often allowing surgery to be deferred. The transforaminal epidural steroid injection has been shown by both prospective and retrospective studies to be beneficial in this clinical setting (1,2). These injections are usually performed by interventional radiologists or pain medicine specialists; however, the management of complications falls more under the purview of neurologists and neurosurgeons.
Review of the literature revealed 2 reports of cervical spinal cord infarction, occurring within 10 minutes after completion of the transforaminal epidural steroid injection (5,6). Both incidents occurred after a C6 injection, 1 after a left-sided injection and 1 after a right-sided injection. Our patient had 2 left-sided injections, 1 at C6 and 1 at C7; symptom onset occurred 30 minutes postprocedure. Clinically, our patient presented in a similar fashion to the others, with lower extremity plegia and variable degrees of upper extremity paresis.
It is notable that the 2 prior cases and our patient all had clinical signs of spinal cord dysfunction above the level of injection that was confirmed using clinical and radiographic examination. In the largest series analyzing patients presenting with a spinal cord infarction, Cheshire et al (7) reported that 8 of 14 MRIs performed “acutely” were normal; on follow-up imaging, there was interval development of focal swelling and increased T2 signal within the spinal cord.
The spinal cord is perfused by an anterior spinal artery and paired posterior spinal arteries; all receive radicular anastomoses that vary in number and caliber (8). Many spinal cord stroke syndromes that have been described correspond to these spinal vessels and manifest clinical signs reflecting their vascular territory. Interestingly, an infarction due to interruption of a spinal radicular artery is clinically indistinguishable from the anterior spinal artery syndrome (7). In a prospective study, it has been demonstrated that during the transforaminal epidural steroid injection, fluoroscopically confirmed venous or arterial intravascular contrast injections occur 19.4% of the time (9). Foraminal epidural steroid injections are typically carried out using fluoroscopic guidance. Anatomically, the cannulation of either the anterior or posterior spinal artery is unlikely; rather, cannulation of the radicular artery is more likely. Given an incidence of 19.4%, the paucity of reports in the literature of this devastating complication may reflect either underreporting of complications or rarity of this complication despite intravascular injection, due to interpatient variability in spinal cord vascular anatomy. If this were the case, the report by Tiso et al (10), which hypothesized that steroid crystals are the cause of vascular emboli, thus infarction, may need to be reconsidered.
Whether the patient's previous anterior cervical discectomy and fusion procedure increased the likelihood of this complication is difficult to assess. Given the ventral location of radicular arteries with respect to the nerve root within the foramina, it is certainly plausible that an anterior cervical discectomy procedure could alter the local anatomy, especially if the posterior longitudinal ligament were opened during the procedure. However, the transforaminal epidural steroid injection is performed from a posterior approach, and the injection needle is targeted to the dorsal aspect of the foramina. In addition, it is also likely that the initial injection of the Isovue contrast would help in the recognition of any anomalous anatomy that is not conducive to proceeding with the transforaminal injection.
The management options of spinal cord infarction are not clearly defined; hence, we extrapolated data from the spinal cord injury and stroke literature. Clearly, the immediate consideration in this situation was the elimination of a compressive lesion such as an epidural hematoma. In 1 of the 2 prior reports and in our patient, steroids were administered acutely: methylprednisolone (dose not specified) in the report by Brouwers et al (5) and 10 mg dexamethasone in ours. The neurology literature does not support the use of steroids in cases of acute stroke, as demonstrated in a double-blind controlled trial of high-dose dexamethasone (11). Additionally, there are no data on the use of steroids in the setting of spinal cord infarction, due to its low incidence and a lack of controlled trials. In the spinal cord injury literature, there is evidence that administration of intravenous methylprednisolone within 8 hours of injury results in improved outcome at 6 weeks, 6 months, and 1 year (12,13). However, the inclusion criteria for this trial did not include patients with spinal cord infarction. At the time of transfer to our institution, the patient had already received 48 hours of parenteral dexamethasone and had recovered some neurological function. After weighing the potential side effects of the use of high-dose exogenous steroids with its unknown benefit in this clinical setting, we elected to discontinue the dexamethasone.
It is well known that periods of hypotension are detrimental to neurological recovery in those with spinal cord injury, closed head injury, and cerebral infarction (14–16). There is additional evidence that aggressive management of hemodynamic parameters using volume augmentation and elevation of the mean arterial blood pressure improves neurological outcome after spinal cord injury (16). This pilot study did not include patients with spinal cord infarction; however, we extrapolated the data from the spinal cord injury literature and volume-resuscitated the patient using crystalloid solution to maintain a euvolemic state and to achieve a mean arterial blood pressure goal of greater than 85 mmHg. Although this was accomplished without the need for vasoactive agents, other situations could require augmentation of volume expansion using vasopressors.
Although the patient did not recover neurological function during her inpatient hospitalization at Northwestern, on 8-week follow-up, she had gained strength in both upper extremities and in her right lower extremity, and she is now ambulating independently using a walker (ASIA-D). In the report by Cheshire et al (7), motor improvement occurred during a 2- to 4-week period after a spinal cord infarction, with 42% of the patients demonstrating neurological gains classified as either “improved” or “markedly improved.” In the 2 prior case reports, 1 report did not include neurological follow-up, while in the other report the patient made no motor-function recovery from a status of ASIA-A complete tetraplegia, although his posterior column function did return at 14 days follow-up. Our report is the first to demonstrate motor and sensory recovery in a patient suffering a spinal cord infarction secondary to a cervical transforaminal epidural steroid injection.
CONCLUSION
Cervical radiculopathy and axial pain are very common conditions whose management may include invasive procedures such as the transforaminal epidural steroid injection. Although the incidence of neurological dysfunction secondary to this procedure is low, the consequence can be devastating. Whether a history of previous surgery should prompt further testing for risk stratification has not been studied or validated. There is no current consensus on the management of infarctions of the spinal cord, but data have been extrapolated from the cerebral infarction and spinal cord injury literatures. The use of high-dose steroids and augmentation of mean arterial blood pressure may have facilitated this patient's neurological recovery, although this evidence is anecdotal. However, early recognition of this complication, combined with expedited exclusion of a surgical lesion and avoidance of secondary injury due to hypoxia and hypotension are felt to be important factors in maximizing neurological recovery.
REFERENCES
- Bush K, Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J. 1996;5:319–325. doi: 10.1007/BF00304347. [DOI] [PubMed] [Google Scholar]
- Slipman CW, Lipetz JS, Jackson HB, Rogers DP, Vresilovic EJ. Therapeutic selective nerve root block in the nonsurgical treatment of atraumatic cervical spondylotic radicular pain: a retrospective analysis with independent clinical review. Arch Phys Med Rehabil. 2000;81:741–746. doi: 10.1016/s0003-9993(00)90104-7. [DOI] [PubMed] [Google Scholar]
- Molloy RE, Benzon HT. Interlaminar epidural steroid injections for lumbosacral radiculopathy. In: Benzon HT, Raja SN, Molloy RE, Liu SS, Fishman SM, editors. Essentials of Pain Medicine and Regional Anesthesia. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 331–340. eds: [Google Scholar]
- Benzon HT. Selective nerve root blocks and transforaminal epidural steroid injections for back pain and sciatica. In: Benzon HT, Raja SN, Molloy RE, Liu SS, Fishman SM, editors. Essentials of Pain Medicine and Regional Anesthesia. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 341–347. [Google Scholar]
- Brouwers PJ, Kottink EJ, Simon MA, Prevo RL. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397–399. doi: 10.1016/S0304-3959(00)00437-1. [DOI] [PubMed] [Google Scholar]
- Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection. Spine. 2005;30:E266–E268. doi: 10.1097/01.brs.0000162401.47054.00. [DOI] [PubMed] [Google Scholar]
- Cheshire WP, Santos CC, Massey EW, Howard JF., Jr Spinal cord infarction: etiology and outcome. Neurology. 1996;47:321–330. doi: 10.1212/wnl.47.2.321. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Core Text of Neuroanatomy. 4th ed. Baltimore, MD: William & Wilkins; 1991. pp. 434–438. [Google Scholar]
- Furman MB, Giovanniello MT, O'Brien EM. Incidence of intravascular penetration in transforaminal cervical epidural steroid injections. Spine. 2003;28:21–25. doi: 10.1097/00007632-200301010-00007. [DOI] [PubMed] [Google Scholar]
- Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4:468–474. doi: 10.1016/j.spinee.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Norris JW, Hachinski VC. High dose steroid treatment in cerebral infarction. Br Med J (Clin Res Ed) 1986;292:21–23. doi: 10.1136/bmj.292.6512.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Jr, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Jr, et al. Methylpred-nisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- Subach BR, Marion DW. Intensive care management of cranial trauma. In: Batjer HH, Loftus CM, editors. Textbook of Neurological Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 2871–2879. [Google Scholar]
- Sulter G, Elting JW, Langedijk M, Maurits NM, DeKeyser J. Admitting acute ischemic stroke patients to a stroke care monitoring unit versus a conventional stroke unit: a randomized pilot study. Stroke. 2003;34:101–104. doi: 10.1161/01.str.0000048148.09143.6c. [DOI] [PubMed] [Google Scholar]
- Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure measurement. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]


