Abstract
Background/Objective:
Although the impact of secondary conditions after spinal cord injury (SCI) on health, well being, and financial burden have been studied, there are psychometrically sound scales of secondary conditions in the extant literature. The use of such scales allows for cross-sample comparison of secondary condition prevalence rates and associations with functional, medical, and psychosocial factors. Thus, the purpose of this study was to evaluate the preliminary reliability of a SCI secondary conditions scale.
Methods:
The Spinal Cord Injury Secondary Conditions Scale (SCI-SCS) is a 16-item scale based on the Seekins Secondary Conditions Scale. Sixty-five individuals with SCI completed written surveys at 5 time-points over 2 years.
Results:
Internal consistency across each of the time-points exceeded 0.76; test–retest reliability ranged from 0.569 to 0.805. Convergent validity was assessed with 6 physical functioning items from the SF-12. Spearman ( coefficients were all statistically significant and ranged from 0.317 (accomplished less because of health problems) to 0.644 (pain). The most prevalent secondary conditions were chronic pain, joint and muscle pain, and sexual dysfunction.
Conclusions:
Preliminary testing of the SCI-SCS suggests that it is a reliable and valid scale, and further development (ie, factor analysis, item revision) and examination of validity are recommended with larger and more diverse SCI samples.
Keywords: Spinal cord injuries, Secondary conditions, Respiratory disease, Pain, Spasticity, Urinary tract infections, Pressure ulcers, Autonomic dysreflexia, Psychometrics, Validity, Reliability, Scales
INTRODUCTION
Secondary conditions after spinal cord injury (SCI) have the potential to dramatically impact, not only health, but emotional well being, community participation, and quality of life (1–5). Although life expectancies have risen in recent decades, people with SCI still die at younger ages than the general population because of medical complications and secondary conditions (6). A variety of factors impacting the development of secondary conditions ranging from injury-related characteristics such as level/completeness of and time since injury (7–10) and functional capacity (7,11), to personal characteristics, such as age (8), risk-taking behavior, and substance abuse (12), to larger forces such as sociopolitical, cultural, and health care systems (4,9,13).
Some of the most common secondary conditions include pressure ulcers (7,10,11,14) respiratory problems (eg, pneumonia) (11,15), genitourinary problems (eg, urinary tract infections [UTIs]) (10,16), spasticity (9,10,17,18), pain (17,19,20), and autonomic dysreflexia (10,17) Respiratory complications (9,11,21) and diseases of the genitourinary and skin systems (7,8,11) are among the most frequent reasons for rehospitalization. Rates of rehospitalization caused by secondary conditions can outpace the general population by as much as 2.6 times, which was recently reported in a case-controlled study conducted in Canada (21). Also, secondary conditions do not exist in isolation but have the potential to exacerbate each other, creating a synergistic effect that can lead to serious health complications, and in some cases, death.
In an analysis of cause of death among 29,239 cases in the National SCI Statistical Center database and Shriner's Hospitals' SCI units in the United States, respiratory and heart-related causes accounted for more than one half of the deaths (28% and 23%, respectively) (22). Soden et al (23) examined causes of death in an Australian sample of 195 individuals with SCI who had died up to 19 years after injury. Death from septicemia, pneumonia, disease of urinary system, and suicide were significantly higher than in the general population and were among the leading causes of death after SCI.
In addition to the health burdens, the financial burden of treating secondary conditions after SCI can be substantial. In a prospective study of the financial cost of traumatic SCI, Johnson et al. (24) estimated that 32% of medical costs in the first 2 years after injury were directly attributable to secondary medical complications. Complications in neurologic, skin, respiratory, and orthopedic body systems were the most expensive to treat. In particular, pressure ulcers, although not as frequent a cause of hospitalization as respiratory complications in some studies, lead to substantially longer length of stays, resulting in higher treatment costs (7,8). Loss of employment also adds to the financial burden caused by secondary conditions (11).
Although the impact of secondary conditions on these various life domains have been well studied, there are no psychometrically sound scales designed to measure their overall impact on the health of individuals with SCI. Instruments of this sort are not meant to replace clinical examination but have value in research as a summary index facilitating cross-sample comparisons of the influence of secondary conditions as a whole, as well as the testing of the association of secondary conditions to other functional, medical and psychosocial factors. Thus, the purpose of this study was to evaluate the preliminary reliability and validity of an adapted version of the Seekins Secondary Conditions Questionnaire (SCQ) (25), the Spinal Cord Injury Secondary Conditions Scale (SCI-SCS).
METHODS
Sample and Data Collection
The data used to test the SCI-SCS were drawn from a larger study to evaluate the effectiveness of a holistic health promotion program after SCI and are described elsewhere (26). Inclusion criteria for the holistic study included having a C5 level of injury and below, being 18 to 80 years of age, and being at least 1 year after injury. Completeness and level of injury are based on the International Standards for Neurological Classification of Spinal Cord Injury, developed by the American Spinal Injury Association (ASIA) and International Medical Society of Paraplegia (27). Exclusion criteria included the presence of associated cognitive deficits preventing learning and carryover (ie, traumatic brain injury, dementia), medical problems that could impose a health risk (eg, recent myocardial infarction), and a primary disability that was not caused by SCI.
Data were collected from study participants at 5 time-points during the course of the holistic study, and subsequent follow-up using written surveys was made up of standardized scales and individual items. Time 1 refers to baseline, Time 2 refers to directly after a health promotion intervention, Time 3 refers to a 4-month follow-up, Time 4 refers to the 1-year post-intervention follow-up, and Time 5 refers to a 2-year post-intervention follow-up. The study sample included 65 participants at Time 1 and decreased to 55, 45, 42, and 35 participants, respectively, at Times 2 to 5.
Scale Modification
Description of Original Scale and Rationale for Modification
The SCQ (25) was originally developed to assess secondary conditions associated with chronic disability and their impact on multiple life domains. It is one of the few scales to comprehensively assess the range of possible health problems associated with disability; the SCQ also includes psychological problems, accessibility and mobility problems, and other problems such as medication side effects, alcohol and drug abuse, and social isolation resulting from disability. The SCQ contains 40 items that are rated for the degree to which they limited activity or independence in the prior 3 months. The SCQ has been used in the context of SCI by Krause to examine prevalence of secondary conditions in association with aging (26).
Although the SCQ is quite comprehensive in its approach to the impact of secondary conditions on many facets of life, this broadness limits its usefulness for studies targeting specific conditions or an aggregate of overall severity (through self-report). Second, there is no established scoring scheme or algorithm for the SCQ, which limits its useful in research, specifically in regard to cross-sample comparison.
Item Selection
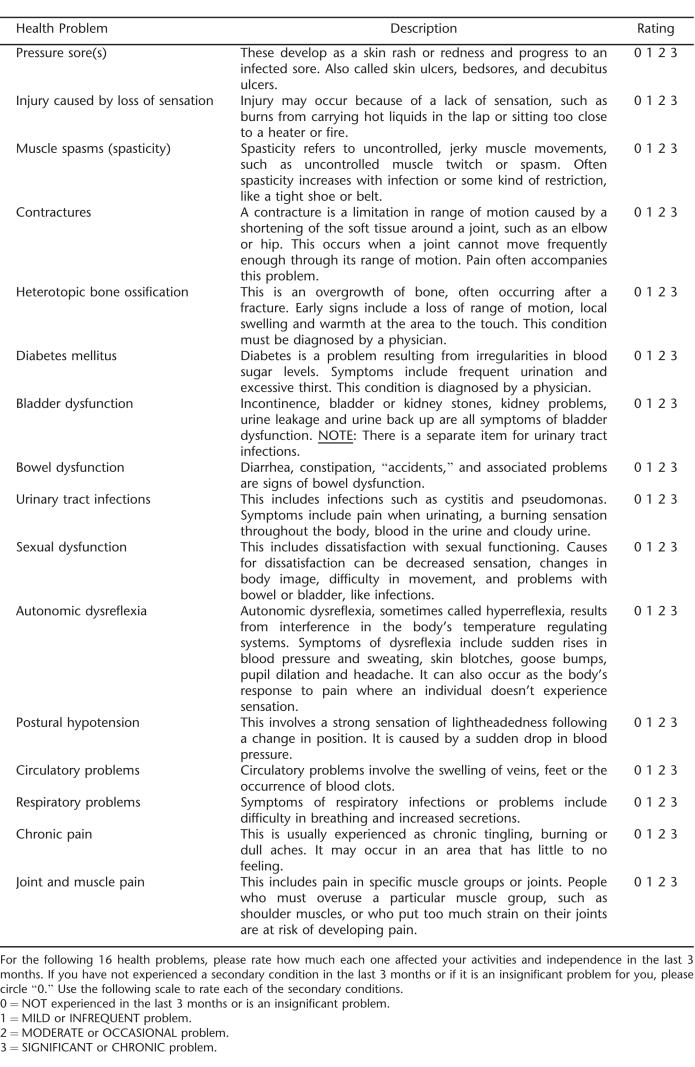
Items for the SCI-SCS were selected from the SCQ to specifically target secondary conditions associated with SCI that directly and indirectly impact health and physical functioning. Items were selected based on 3 criteria: (a) that they represent conditions that are physiologic in nature (vs psychological or environmental); (b) that they are measurable by patient history and physical examination, reported episodes, validated scales, or medical tests or interventions; and (c) those that can be either prevented or managed with medical intervention and/or health behaviors. Using this criteria, 16 items were selected from the original 40 items, representing problems in the areas of skin, musculoskeletal, pain, bowel/bladder, and cardiovascular; these are briefly described below.
Pressure ulcers are a very serious, costly, and ever present complication after SCI and have a significant impact on morbidity and mortality (28). Pressure ulcers come at significant expense and can take months to years to heal, requiring multiple surgeries and wound care. A number of secondary conditions, including autonomic dysreflexia, increased spasticity, osteomyelitis, and infection are also related to pressure ulcers, broadening its detrimental effects.
Bladder dysfunction after SCI can affect individuals in numerous ways including urinary incontinence, detrusor sphincter dyssynergia, and bladder and kidney stones. Both symptomatic and asymptomatic infections, often requiring hospitalization, are also very prevalent after SCI (29). Such complications, including upper tract dysfunction, may lead to the most serious and potentially fatal condition of kidney failure (30).
Autonomic dysreflexia is defined as an unopposed sympathetic nervous system discharge caused by a noxious or intense stimulus below the level of injury (31). This is primarily seen in injuries at T6 and above, and prolonged and elevated blood pressure can lead to a hypertensive crisis, possibly causing stroke, myocardial infarction, retinal hemorrhages, and even death.
Paralysis, a hypercoagulable state, and injury to blood vessel lining all can contribute to the development of a deep vein thrombosis (DVT) after SCI. The most serious complication of DVT is pulmonary embolism, causing severe cardiopulmonary compromise or even death. Other complications from DVT included leg edema and pain.
The precise mechanisms and etiologies of pain in SCI are not well understood. Chronic pain can be nociceptive in nature and related to an injury (eg, multiple fractures, surgical sites) or neuropathic. Neuropathic or “central pain,” although largely misunderstood, is believed to be primarily related to injuries to the spinal cord or peripheral nerves (32). Interference by pain can range from none in everyday activities to totally disabling and can have a dramatic impact physically and emotionally.
Long-term wheelchair and other assistive device users often face overuse injuries to the shoulders and other upper extremity joints (33), with resulting arthritis, bursitis, tendonitis, and common nerve entrapments (eg, ulnar neuropathy, carpal tunnel syndrome) that can further restrict mobility.
Pulmonary complications, especially in those with tetraplegia, arise primarily from the inability to produce an effective cough to clear secretions, generate deep breaths to keep peripheral airways open, and hypoventilation (34). Diminished pulmonary reserve can make respiratory infections such as pneumonia much more serious in individuals with SCI.
Growing evidence is supporting the higher incidence of diabetes and glucose intolerance in individuals with SCI compared with the general population (35). Disuse of large muscle groups in the legs is a factor in altered glucose metabolism. Long-term effects of diabetes, especially when untreated, can significantly impair resistance to infection, retard healing of pressure ulcers and other wounds, and increase the risk of coronary artery and peripheral vascular disease.
Spasticity is a condition of velocity-dependent increase in tonic muscle stretch reflexes and is quite prevalent in SCI. This can hinder proper positioning in a wheelchair and cause pain and difficulty with performing regular hygiene. Some may actually use spasticity for functional activities such as transfers or standing, whereas others may have severe limitations from increased muscle tone (36).
Bowel dysfunction after SCI has been found by many to be a major problem. Fear of bowel accidents can lead to limited community participation. Constipation and bowel impaction can also be a cause of autonomic dysreflexia.
The introduction of catheters, especially indwelling Foley or suprapubic catheters, can introduce bacteria resulting in infection. In fact, over time, most individuals with SCI will develop colonization of the urinary tract with bacteria. Urinary incontinence and increased spasticity may also occur with UTIs. A more serious infection may also occur, resulting in fever, chills, rigors, and systemic infections (38).
Postural hypotension, sometimes referred to as “orthostasis,” is caused by a relative loss of sympathetic tone after SCI. Effects of this hypotension may range from no clinical affect to significant lightheadedness and syncope.
Impaired or absent sensation below the level of the injury make individuals with SCI much more susceptible to injuries of the skin and extremities. After injury, loss of bone mass below the level of the injury also increases the risk of fractures (39). An inconspicuous leg injury may lead to fractures, some of which may go undetected. These injuries can range from being minor to very serious complications.
Over time, joints that do not go through a regular range of motion may tighten up, and as connective tissues around a joint become more firm, contractures will develop. These contractures may not always significantly impact the individual's functional level, but have the potential to restrict mobility.
Abnormal bone formation around joints below the level of the injury are most common in the hips, knees, elbows, and shoulders, although the precise mechanism of why this occurs is not well understood (40). If the heterotopic ossification is severe, range of motion and positioning may be impaired, with an increased risk of pressure ulcers in the region of heterotopic ossification.
Sexual function after SCI differs greatly between men and women with SCI. Men will almost always have erectile and ejaculatory dysfunction. In women, the regular menstruation cycle is usually absent temporarily, but will return in 90% of individuals within 6 to 12 months. In women, sexual dysfunction may include dryness of the vagina during intercourse.
Scoring
The rating scale uses a 4-point ordinal scale ranging from 0 (not experienced/insignificant problem never limiting activity) to 3 (significant/chronic problem). Total scores range from 0 to 48 and are derived from the sum of the problem ratings such that higher scores indicate greater overall problems with secondary conditions. The SCI-SCS is given in Appendix A.
Convergent Validity
The SF-12 is an abbreviated version of the widely used SF-36 (41), a widely used measure of health-related quality of life. The SF-12 has been used in several studies with SCI populations (42–45), and version 1 of the SF-12 was also used by the Model SCI Systems from 1995 to 2000. Twelve items reflect physical and psychological functioning and pain and the degree to which these limited activity in the previous 4 weeks, as well as items related to psychological well being. In the holistic study from which these data were drawn, physical functioning items that referred to “climbing stairs” were modified to read “stairs/ramps.” Six items related to physical functioning, general health, and pain were used to test convergent validity. Items were used rather than the total Physical Functioning score because, in computing that score, mental health functioning items are included, and these were not of interest in this study. Additionally, because this is the first exploration of this scale, we wanted to more closely examine how well it converged with specific physical functioning domains.
Two items refer to the ability to carry out moderate activities such as pushing a vacuum cleaner or climbing a short ramp and more intensive activities such as climbing several ramps. These are rated on a scale of 1 (not limited at all) to 3 (limited a lot). Two items refer to the degree that physical health had interfered with the ability to carry out work or regular daily activities. These are rated on a scale of 1 (all of the time) to 5 (none of the time). One item refers to the degree that pain interfered with normal home and vocational activities. Finally, a single item assessed self-rating of health in general rated on a scale of 1 (poor) to 4 (excellent).
Statistical Procedures
Before analyses were performed, data were checked for skewness; the total SCI-SCS score was not significantly skewed. Participants' responses at Time 1, for which there were complete data and the largest sample size. The analyses used were aimed at developing a brief measure of secondary conditions that possesses acceptable psychometric characteristics. To that end, we examined 2 aspects of reliability: (a) internal consistency (Cronbach α) and (b) test–retest reliability. Acceptable levels of internal consistency are 0.70 (46) or greater and insure that measurement error is minimized. Furthermore, we conducted item analyses to insure that all items had acceptable item total correlations (r ≥ 0.20). Acceptable levels of test–retest reliability vary depending on the construct and the time intervals involved. Although few guidelines exist for interpreting test–retest coefficients, typically those evaluated for time periods closer together will be higher than for those evaluated for time periods further apart. Given that our data collection time periods span just a few months to 2 years, it is likely that there will be some variability in the test–retest coefficients. Nevertheless, we expect a fair degree of stability over time (r ≥ 0.50), because most of the conditions of interest are typically chronic in nature.
We also evaluated 2 types of validity: content and construct. Our content validity analysis was based in the selection of items from the larger pool of items representing secondary conditions. These procedures are detailed above, and the rationale for inclusion of each item is detailed in the measures section. Our intent was to create a content-valid measure that assessed a subset of common and important secondary conditions that directly and indirectly impact health and well being. Our construct validity analyses are made up of Spearman ρ correlations between our measure of secondary conditions and physical functioning, general health rating, and pain SF-12 items. We expected the secondary conditions scale, given its strong emphasis on physical conditions, to show convergent validity with these items.
RESULTS
Demographic and Injury Characteristics
The sample was predominantly male, white, and generally well educated with a mean age of 43.8 ± 13.4 (SD) years. Slightly less than one half were married, and a third were employed either full- or part-time. Participants were 13.7 ± 11.0 years postinjury on average and nearly evenly divided between paraplegic and tetraplegic injuries; approximately two thirds had complete injuries. Complete demographic and injury characteristics of the sample are provided in Table 1.
Table 1.
Demographic Characteristics of the Sample (N = 65)
Internal Consistency and Test–Retest Reliability
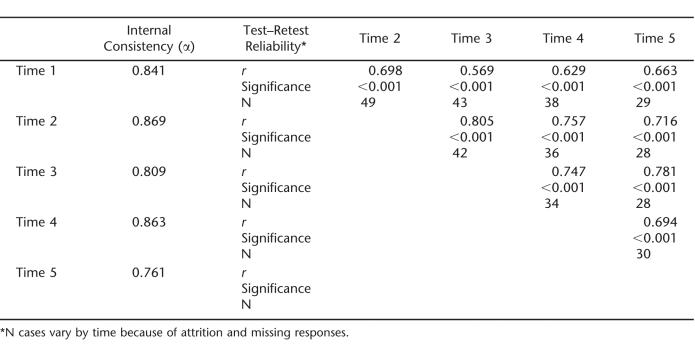
Internal consistency coefficients and test–retest reliability coefficients of the SCI-SCS at each time-point are given in Table 2. Internal consistency exceeded 0.80 with the exception of Time 5, which was still above the acceptable threshold at 0.761. Results of item analyses (Time 1) revealed that item total correlations ranged between 0.26 and 0.65. As a result, all items were retained to compute the total SCI-SCS score. Test–retest reliability coefficients generally exceeded 0.60, suggesting generally acceptable reliability across time.
Table 2.
Internal Consistency and Test–Retest Coefficients of SCI-SCS total score (T1–T5)
Prevalence of and Degree of Problem with Secondary Conditions in the Sample
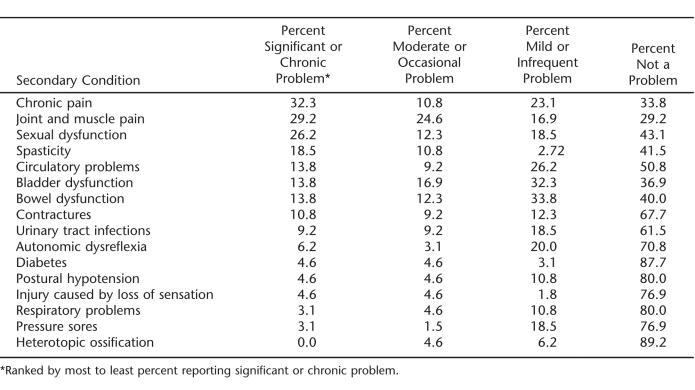
Using Time 1 data, on average, the sample reported some degree of problem with an average of 6 ± 3 secondary conditions. As shown in Table 3, chronic pain, joint and muscle pain, and sexual functioning were most often reported as being most significant or chronic problems. There was no statistically significant difference between injury level/completeness on the SCI-SCS total score (F3,58 = 2.57, P = 0.063).
Table 3.
Proportion of Sample Reporting Degree of Problems with Secondary Conditions (Time 1, N = 65)
Convergent Validity
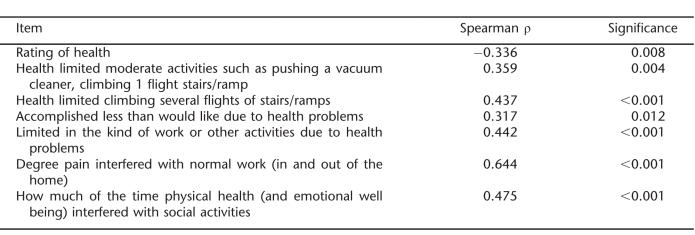
As shown in Table 4, there were significant correlations between the SCI-SCS total score and the 6 SF-12 items described above. Most associations were moderate, ranging from 0.317 to 0.644 and in expected directions.
Table 4.
Spearman Correlation Coefficients–SCI-SCS Total Score and Physical Function Items
DISCUSSION
The findings of this first examination of the SCI-SCS suggest that it is a reliable measure, being both internally consistent and having good test–retest reliability. In addition, examination of convergent validity with respect to physical functioning suggested preliminary support for its validity. The association of the SCI-SCS total score association with aspects of physical function were significant and in expected directions. Its strongest association was with pain, which was expected, given chronic pain and joint and muscle pain were most often reported as problematic for the sample, and is consistent with the literature that pain is a significant and pervasive secondary condition in SCI (43,47,48).
Future Directions for Scale Development
The psychometric properties of this scale suggest promise for its further development using larger and more diverse SCI samples. Future development of this scale is warranted in terms of its items, their description, and response scales. Examining the SCI-SCS's factor structure in a larger sample is an important step in scale development. Rephrasing of item descriptions may also be considered for particular items, such as sexual dysfunction, which reflects satisfaction rather than dysfunction. Because retrospective data were used in this study, items could not be reworded before data collection took place but should be considered in future iterations of the scale to clarify some items. Finally, further examination of the scale's construct validity using, for example, correspondence between clinical examination and response to the scale items would be worthwhile to pursue in future studies. More broadly, the use of such a standardized scale in general and an aggregate score in particular should be weighed by SCI investigators in future studies.
Limitations
One of the major limitations of using archival data is lack of control over variables selected for measurement or the ability to modifying items or rating schemes before administration. Furthermore, the context of the data used in this analysis also is a potential source of bias such that individuals with interest in wellness and improving their health behavior participated in the health promotion program. Accordingly, they may not be representative of the larger SCI population in terms of their overall health status, prevalence, and impact of secondary conditions. Some findings may also reflect the characteristics of the sample rather than the sensitivity of the scale. For example, there was no difference in scores by level/completeness of injury; a more diverse sample may highlight the ability (or lack of ability) of the scale to distinguish by injury level or other injury or health characteristics.
CONCLUSIONS
The SCI-SCS is not designed to objectively evaluate secondary conditions in SCI, but rather the subjective experience of these problems for use in research to allow for cross-sample comparison and examination of the association of secondary conditions and various aspects of health and psychosocial well being in people with SCI. Preliminary assessment of the reliability and validity of the SCI-SCS suggests a promising scale for use with larger and diverse SCI samples.
Acknowledgments
We thank Eric Zemper, PhD, for work on this research and Anthony Chiodo, MD, and Joseph Hornyak, MD, PhD, for contributions to the development of this scale.
Appendix A: The Spinal Cord Injury Secondary Conditions Scale
Footnotes
This study was supported by a grant from the University of Michigan Health Systems (UMHS) Venture Investment Fund; by the UMHS General Clinical Research Center (National Institutes of Health grant M01-RR00042); and by the U-M Model SCI Care System, funded by the National Institute on Disability and Rehabilitation Research, Offices of Special Education and Rehabilitation Services, US Department of Education (H133N000009).
REFERENCES
- Dijkers M. Correlates of life satisfaction among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:867–876. doi: 10.1016/s0003-9993(99)90076-x. [DOI] [PubMed] [Google Scholar]
- Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil. 1998;79:1433–1439. doi: 10.1016/s0003-9993(98)90240-4. [DOI] [PubMed] [Google Scholar]
- McColl MA, Arnold R, Charlifue S, Glass C, Savic G, Frankel H. Aging, spinal cord injury, and quality of life: structural relationships. Arch Phys Med Rehabil. 2003;84:1137–1144. doi: 10.1016/s0003-9993(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Ville I, Ravaud J-F. Group Tetrafigap. Subjective well-being and severe motor impairments: the Tetrafigap survey on the long-term outcome of tetraplegic spinal cord injured persons. Soc Sci Med. 2001;52:369–384. doi: 10.1016/s0277-9536(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Boschen KA, Tonack M, Gargaro J. Long-term adjustment and community reintegration following spinal cord injury. Int J Rehabil Res. 2003;26:157–164. doi: 10.1097/00004356-200309000-00001. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center . Spinal Cord Injury: Facts and Figures at a Glance. Birmingham, AL: University of Alabama; 2003. [Google Scholar]
- Savic G, Short DJ, Weitzenkamp D, Charlifue S, Gardner BP. Hospital readmissions in people with chronic spinal cord injury. Spinal Cord. 2000;38:371–377. doi: 10.1038/sj.sc.3101019. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Lim K, Taylor L, Soden R, Rutkowski S. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord. 2004;42:359–367. doi: 10.1038/sj.sc.3101601. [DOI] [PubMed] [Google Scholar]
- Klotz R, Joseph PA, Ravaud JF, Wiart L, Barat M. the Tetrafigap group. The Tetrafigap survey on the long-term outcome of tetraplegic spinal cord injured persons. Part III. Medical complications and associated factors. Spinal Cord. 2002;40:457–467. doi: 10.1038/sj.sc.3101317. [DOI] [PubMed] [Google Scholar]
- Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury—a population-based study. Am J Phys Med Rehabil. 2000;79:526–535. doi: 10.1097/00002060-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Hawkins DA, Heinemann AW. Substance abuse and medical complications following spinal cord injury. Rehabil Psychol. 1998;43:219–231. [Google Scholar]
- McColl MA, Charlifue S, Glass C, Savic G, Meehan M. International differences in ageing and spinal cord injury. Spinal Cord. 2002;40:128–136. doi: 10.1038/sj.sc.3101264. [DOI] [PubMed] [Google Scholar]
- McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 Southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Leoni MEG, De Ruz AE. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9:780–785. doi: 10.1046/j.1469-0691.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36:45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Barrett H, McClelland JM, Ruthowski SB, Siddall PJ. Pain characteristics in patients admitted to hospital with complications after spinal cord injury. Arch Phys Med Rehabil. 2003;84:789–795. doi: 10.1016/s0003-9993(02)04944-4. [DOI] [PubMed] [Google Scholar]
- Charlifue SW, Weitzenkamp D, Whiteneck GG. Longitudinal outcomes in spinal cord injury: aging, secondary conditions, and well-being. Arch Phys Med Rehabil. 1999;80:1429–1434. doi: 10.1016/s0003-9993(99)90254-x. [DOI] [PubMed] [Google Scholar]
- Dryden DM, Saunders LD, Rowe BH, et al. Utilization of health services following spinal cord injury: a 6-year follow-up study. Spinal Cord. 2004;42:513–525. doi: 10.1038/sj.sc.3101629. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38:604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Brooks CA, Whiteneck GG. Cost of traumatic spinal cord injury in a population-based registry. Spinal Cord. 1996;34:470–480. doi: 10.1038/sc.1996.81. [DOI] [PubMed] [Google Scholar]
- Seekins T. Secondary Conditions Questionnaire. University of Montana; 1990. [Google Scholar]
- Krause JS. Aging after spinal cord injury: an exploratory study. Spinal Cord. 2000;38:77–83. doi: 10.1038/sj.sc.3100961. [DOI] [PubMed] [Google Scholar]
- American Spinal Injury Association . American Spinal Injury Association, International Medical Society of Paraplegia; 2001. International Standards for Neurological and Functional Classification of Spinal Cord Injury. [Google Scholar]
- Consortium of Spinal Cord Medicine Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24(suppl 1):S40–S101. doi: 10.1080/10790268.2001.11753592. [DOI] [PubMed] [Google Scholar]
- Bodner D. Urologic management in spinal cord injury. In: Lin V, editor. Spinal Cord Medicine: Principles and Practice. New York, NY: Demos Medical Publishing; 2003. pp. 299–306. [Google Scholar]
- Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F. Survival and cause of death after traumatic spinal cord injury. A long-term epidemiological survey from Denmark. Spinal Cord. 1997;35:76–85. doi: 10.1038/sj.sc.3100351. [DOI] [PubMed] [Google Scholar]
- Teasell R, Arnold J, Krassioukov A. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- Yezierski R. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;68:185–194. doi: 10.1016/s0304-3959(96)03178-8. [DOI] [PubMed] [Google Scholar]
- Subbarao J, Klopfstein J, Turpin R. Prevalence and impact of wrist and shoulder pain in patients with spinal cord injury. J Spinal Cord Med. 1995;18:9–13. doi: 10.1080/10790268.1995.11719374. [DOI] [PubMed] [Google Scholar]
- Peterson W. Pulmonary management of spinal cord injury. In: Kirshblum S, Campagnolo D, DeLisa J, editors. Spinal Cord Medicine. Philadelphia, PA: Lippincott, Williams and Wilkins; 2002. pp. 135–154. [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid-metabolism in veterans with paraplegia or quadriplegia—a model of premature aging. Metab Clin Exp. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Levi R, Hultling C, Nash MS, Seiger A. The Stockholm Spinal Cord Injury Study: 2. Associations between clinical characteristics and post-acute medical problems. Paraplegia. 1995;33:585–594. doi: 10.1038/sc.1995.125. [DOI] [PubMed] [Google Scholar]
- Consortium of Spinal Cord Medicine Clinical Practice Guidelines: neurogenic bowel management in adults with spinal cord injury. J Spinal Cord Med. 1998;21:248–293. doi: 10.1080/10790268.1998.11719536. [DOI] [PubMed] [Google Scholar]
- Linsenmeyer T. Neurogenic bladder following spinal cord injury. In: Kirshblum S, Campagnolo DI, DeLisa J, editors. Spinal Cord Medicine. Philadelphia, PA: Lippincott, Williams & Wilkins; 2002. pp. 181–206. [Google Scholar]
- Bauman WA, Garland D, Schwartz E. Calcium metabolism and osteoporosis in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 1997;2:84–95. [Google Scholar]
- Banovac K, Sherman A, Estores I, Banovac F. Prevention and treatment of heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2004;27:376–382. doi: 10.1080/10790268.2004.11753775. [DOI] [PubMed] [Google Scholar]
- Ware J. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- Putzke JD, Barrett JJ, Richards JS, DeVivo MJ. Age and spinal cord injury: an emphasis on outcomes among the elderly. J Spinal Cord Med. 2003;26:37–44. doi: 10.1080/10790268.2003.11753659. [DOI] [PubMed] [Google Scholar]
- Putzke JD, Richards JS, Dowler RN. The impact of pain in spinal cord injury: a case-control study. Rehabil Psychol. 2000;45:386–401. [Google Scholar]
- Putzke JD, Hicken BL, Richards JS. Race: predictor versus proxy variable? Outcomes after spinal cord injury. Arch Phys Med Rehabil. 2002;83:1603–1611. doi: 10.1053/apmr.2002.35115. [DOI] [PubMed] [Google Scholar]
- Hicken BL, Putzke JD, Richards JS. Bladder management and quality of life after spinal cord injury. Am J Phys Med Rehabil. 2001;80:916–922. doi: 10.1097/00002060-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain. 2002;100:231–242. doi: 10.1016/S0304-3959(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury—relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004;42:665–673. doi: 10.1038/sj.sc.3101641. [DOI] [PubMed] [Google Scholar]