Abstract
Summary:
This special report traces the path of spinal cord injury (SCI) from ancient times through the present and provides an optimistic overview of promising clinical trials and avenues of basic research. The spinal cord injuries of Lord Admiral Sir Horatio Nelson, President James A. Garfield, and General George Patton provide an interesting perspective on the evolution of the standard of care for SCI. The author details the contributions of a wide spectrum of professionals in the United States, Europe, and Australia, as well as the roles of various government and professional organizations, legislation, and overall advances in surgery, anesthesia, trauma care, imaging, pharmacology, and infection control, in the advancement of care for the individual with SCI.
Keywords: Spinal cord injuries; Spinal cord medicine; History; Munro, Donald; Guttman, Ludwig; Young, John; Bors, Ernest; Comarr, A. Estin; Rossier, Alain
INTRODUCTION
As with most other topics, in order to acquire a complete understanding of spinal cord injury (SCI), one must appreciate the events that comprise its past, present, and future. This “trinity of time” for SCI has a most interesting past, an exciting present, and a very promising future.
THE PAST—AN AILMENT NOT TO BE TREATED
Nowadays, in science as in industry, much emphasis is placed on the future and the shaping of the present so that it leads directly to some desired outcome. Often overlooked in the planning of such quests are the events that have preceded them and how they have shaped the present. Understanding those events can give one a better grasp of the reasons that justify one's pursuits. We tend to forget the admonition of the well-known Spanish philosopher, George Santayana (1863–1952) who while teaching at Harvard University in the US said, “Progress, far from consisting in change, depends on retentiveness…. Those who cannot remember the past are condemned to repeat it.” (1) This advice is especially worth remembering when it comes to SCI, which actually has a rich, absorbing past that harks back a long way to roughly 2,500 years BCE. We know this from the writings inscribed in the Edwin Smith surgical papyrus.
Little if any time is given to this important treatise during any of the levels of medical education, ie, medical school through residency and graduate medical education. Yet, as Trevor Hughes explains in his analysis of this document, it is (a) the first known record extant that can be called a scientific document, (b) the first known important medical treatise, (c) the first medical document concerned with trauma, and (d) the first documentation of cases of spinal cord injury (2).
The scroll was purchased by an American Egyptologist, Edwin Smith, in Luxor, Egypt, in 1862 and then translated from the hieratic by James Henry Breasted (Figure 1), at the behest of the New York Historical Society and published in 1930 (3). The document contains descriptions of 48 traumatic cases, 6 involving the cervical spine, and 2 of those 6 are clearly injuries to the spinal cord. The author has an obvious knowledge of anatomy and experience in the surgical treatment of his day. Some have posited the author was Imhotep. The scribe who copied the original document some 1,000 years later (the copy Smith purchased) was also knowledgeable and added comments of his own. The author explains to the reader (his students) how each of the 48 cases should be treated, eg, “packing the wounds with fresh meat.” Of special interest to us are his instructions that those 2 cases of SCI are not to be treated at all—“an ailment not to be treated” (2).
Figure 1. Under the sponsorship of the New York Historical Society, James Henry Breasted translated the Edwin Smith surgical papyrus from the hieratic. The translation was published in 1930. Photo is from the Encyclopedia Britannica online at http://www.britannica.com.

In fairness to the author and his therapeutic nihilism toward SCI, more than 4,000 years ago he was probably dealing with injuries sustained during battlefield conditions where principles of triage prevailed and where limited resources were reserved for those who could be healed and returned to duty. Yet, one cannot help but be saddened that the author's advice not to treat was followed wittingly or unwittingly down through the millennia that ensued, up until the early part of the 20th century. Examples of this nihilistic philosophy can be cited from the records of people familiar to us in the 19th and 20th centuries. Let us examine 3 such well known cases and how they were managed:
1. Lord Admiral Sir Horatio Nelson (1758–1805). There is perhaps no British naval hero more revered by Great Britain than Lord Nelson (Figure 2). Having lost an eye and an arm in the service of his country during the course of his numerous naval victories, he finally lost his life, victorious at the battle of Trafalgar, on October 20, 1805. On deck, urging on his men, boarding the Spanish man-o'-war that came alongside his flagship, HMS Victory, he was felled by a sniper's bullet that entered his chest and spinal cord. He was immediately taken below, and the ship's surgeon was summoned. Lord Nelson told the surgeon, Mr Beatty (in the British Commonwealth, surgeons are addressed as “Mr”), “All power of motion and feeling below my chest are gone.” Mr Beatty duly examined his patient, confirmed his SCI and then, no doubt with head bowed, said “My Lord, unhappily for our country, nothing can be done for you.” One can safely say that if anything could have been done, it surely would have been for Lord Nelson. But Mr Beatty was right. His patient had “an ailment not to be treated” (4).
Figure 2. One example of the therapeutic nihilism toward spinal cord injury care before 1950 is the case of Lord Nelson. At the battle of Trafalgar, October 20, 1805, Lord Nelson was felled by a sniper's bullet that entered his chest and spinal cord. Nothing could be done for him.

Nelson's victory that day destroyed both the French and Spanish armadas and while it did not stop his nemesis, Napoleon, from his victorious march across Europe, it did ensure that Nelson's island country would remain safe from any subsequent attack from the sea by L'Empereur. For this, he is recognized by a monument at Trafalgar Square in London, which dominates its surroundings (5).
2. James A. Garfield (1831–1881), 20th President of the United States. Shortly after taking office, Garfield was shot by a disgruntled, passed-over office seeker. The bullet lodged in the conus medullaris, resulting in paralysis of his legs, bowel, and bladder. Complications ensued and after surviving 80 days, he succumbed (6). Despite the medical treatments that could be brought to bear at that time, President Garfield's physicians, whom some say were more harmful than helpful, accomplished no more than what Mr Beatty was able to do. The 20th President had “an ailment not to be treated.” The Museum of the Armed Forces Institute of Pathology has on display a specimen of the former President's shattered vertebra (Figure 3). (It also has a specimen of the upper cervical vertebrae of John Wilkes Booth, murderer of President Abraham Lincoln, who was shot in the neck when captured) (4).
Figure 3. (A) Illustration of James A. Garfield, 20th President of the United States, who was shot in the conus medullaris and survived 80 days (6). (B) The museum of the Armed Forces Institute of Pathology has on display a specimen of the former president's shattered vertebra. Reproduced with permission from the National Museum of Health and Medicine.
By the time the US entered World War I (April 1917), many combat SCIs had been incurred by the soldiers of the warring parties, and this continued until the armistice (November 11, 1918). The famous American neurosurgeon, Harvey Cushing (1869–1939) (Figure 4), writing from the 14th General Base Hospital in France, reported that “the conditions were such [that] 80% died in the first few weeks [and]…. only those cases survived in which the spinal lesion was a partial one.” The rest had ailments that were not to be (could not be) treated. Cushing and others lamented the defeatist attitude that prevailed at that time toward SCI among all the health care professions (4,7).
Figure 4. Harvey Cushing, famous American neurosurgeon, wrote, “The conditions were such that 80% died in the first weeks [and]… only those cases survived in which the spinal lesion was a partial one.” Photo courtesy of the Cushing Whitney Medical Historical Library, Yale University, New Haven, CT.

3. General George Patton (1885–1945), commander of the US Seventh and then the Third Army during World War II (Figure 5). A volatile yet eminently successful leader throughout the campaigns in North Africa and Europe, he was involved in a motor vehicle crash only months after the conclusion of war in the European theater. He sustained a cervical spinal cord injury, possibly incomplete. Patton knew there was no cure for SCI (an ailment not to be treated). He thus refused all treatment and was reported to have died from a cardiovascular complication while still hospitalized (8).
Figure 5. General George Patton (1885–1945), Commander of the US Seventh and then the Third Army during World War II. He sustained a cervical spinal cord injury in a motor vehicle crash.

Finally, as another example of conditions that prevailed for persons with SCI, even as recently as 1944, there is a poignant memo quoted by Silver: “In the spring of 1944, I was called to group headquarters for an interview with the group officer, a surgeon of formidable character. ‘Allen,’ he said to me, ‘I am sorry to have to inflict this on you, but we have been ordered to open a spinal unit at Leatherhead Hospital and I want you to take charge of it. Of course, as you know, they are hopeless cases—most of them die, but you must do your best for them.’ With these words of ‘encouragement,’ I returned home sadly” (7).
Although a “cure” for SCI has yet to be discovered, it would be wrong to say that no interest in the pursuit of this objective existed prior to the latter part of the 20th century. As we will see, that is when the quest for a cure began to be taken seriously by more and more investigators.
Given their historical role of first responders to trauma, it is not surprising that the first group to express interest in finding some way of ameliorating the effects of SCI was surgeons. It is equally not surprising that their proposed interventions involved surgery. And this mind-set has prevailed even to the present. During the early 19th century, a controversy arose between two British surgeons, Sir Astley Cooper and Sir Charles Bell (Figure 6). The former favored operating on the injured spinal cord, arguing that since death was inevitable anyway without surgery, nothing was to be lost. The latter posited that surgery only increased the risk of death and could further damage nerve fibers with any potential to improve (9).
Figure 6. Left: Sir Astley Cooper favored operating on the injured spinal cord, arguing that since death was inevitable anyway without surgery, nothing was to be lost. Right: Sir Charles Bell posited that surgery only increased the risk of death and could further damage nerve fibers with any potential to improve.

Over the next 100-plus years, the topic of reversing the effects of SCI, when it did appear in the medical literature, was largely centered on the feasibility of operating. This prevailed until the last decade of the 20th century, when pharmacologic treatments made their appearance (10,11). In point of fact, the only reason that the issue of surgery dominated the pursuit of finding a cure for SCI over the years was because of landmark discoveries in other branches of medicine that continued to make surgery on the injured spine (or any part of the body, for that matter), as well as treatment of complications, safer and easier. These breakthroughs were numerous, and a thorough discussion is beyond the scope of this article; however, it is important to remember that the problems that they solved were as much of a challenge to their discoverers in their era as the quest for a cure for SCI is to us today. I will therefore simply list some of them and comment briefly.
In the field of microbiology, Louis Pasteur (1832–1895) advanced our knowledge of sterilization as well as the “germ theory” in general (12); Ignaz Semmelweis (1818–1865) demonstrated to skeptics that hand washing and clean technique could drastically reduce the transmission of disease, and yet he was ridiculed in 1849, even by the most renowned pathologist of his day, Rudolf Virchow (1821–1902), for proposing that invisible organisms could cause illness (13); Joseph Lister (1827–1912) applied the concept of antisepsis to surgery and wound treatment (14); Robert Koch (1843–1910) proved beyond a doubt that microbes cause disease (15); William Stewart Halstead (1852–1922) introduced the surgical glove (16); and Alexander Fleming (1881–1955) discovered penicillin and inaugurated the antibiotic era (17).
Although the contributions of Semmelweis, Lister, and Halstead were breakthroughs for infection prevention, clearly Fleming's discovery and the production of the antibiotics that followed had the greatest impact on survival after SCI. Now, infections that became established during the acute and chronic phases of SCI could be eliminated. Conditions prior to the antibiotic era were described by the medical superintendent at the Royal Star and Garter Home (the first spinal unit in the United Kingdom) in 1934: “Any local infection is liable to be followed by a general infection, cystitis, or pyelitis; and pyelitis is almost always the ‘end condition’ of the paraplegic” (18).
In the field of anesthesia, Sir Humphrey Davy (1778–1829) demonstrated the use of nitrous oxide, William T. G. Morton (1819–1868) the use of ether, and John Snow (1813–1858), the use of chloroform (19). When the senior surgeon at The Massachusetts General Hospital, John Collins Warren (1778–1856), allowed Morton to demonstrate “etherization” on his patient who then felt no pain during the resection of a vascular tumor on the skin over the mandible, he proclaimed with amazement to his colleagues “Gentlemen, this is no humbug” (Figure 7a and 7b). The era of painless surgery had begun (13,20).
Figure 7a. Senior Surgeon at Massachusetts General Hospital John Collins Warren (1778–1856) allowed William T. G. Morton to demonstrate “etherization” on his patient.

Figure 7b. Scene believed to be a re-enactment of the demonstration of ether anesthesia by W. T. G. Morton on October 16, 1846. Mr. Holman with surgeons: John Mason Warren, George Hayward, Solomon D. Town-send, John Collins Warren and James Johnson around man on operating table.
In the field of hematology, Karl Landsteiner (1868–1943) discovered the ABO blood typing system and later he and Alexander Weiner (1907–1976) discovered the Rh system. Through the detection of incompatibilities between patient and blood donor, and thereby the avoidance of hemolytic reactions, surgery was made safer, as were transfusions for any other reason (21).
In the field of imaging, the discovery by William Conrad Roentgen (1845–1923) of the x-ray (22), the application of radiography to myelography by J. A. Sicard (1872–1929) (23), the development of computerized tomography (CT) scanning by William Oldendorf (1925–1992) (24,25), and the development of magnetic resonance imaging (MRI) by Raymond Damadian, A. Reid, and others, all made spinal surgery safer by allowing the surgeon to have more knowledge of the pathology and more preparation prior to operating (26).
Early treatment for spinal injuries included closed and open methods. Spinal traction was described both in the Edwin Smith papyrus and later by Hippocrates (470–410 BC) (27). In modern times, Sir Geoffrey Jefferson (1886–1961) utilized halter traction, while Sir Reginald Watson-Jones (1902–1972) placed the patient in the prone position between 2 tables (28). W. Gayle Crutchfield (1900–1972) first described skeletal traction in 1933 (29,30). In 1955, Vernon Nickel (1918–1993) and colleagues applied the principle of skeletal traction, called halo traction, by using the halo vest (31). These methods provided a way of obtaining closed reduction and of maintaining better alignment of the spine, whether the patient was treated operatively or not.
Finally, the techniques of surgery itself improved, providing better reduction of deformity and stabilization over the last 50 years. If we were to focus on major contributors, one would have to begin with Paul Harrington (1911–1980) (Figure 8) for his introduction of a system of distraction and compression rods and hooks. His system was intended for the treatment of scoliosis (32), but spinal surgeons were quick to recognize its capacity to adapt to the treatment of spinal fractures and dislocations, particularly of the thoracolum-bar spine (33). Many instruments have been developed in the years that followed, most notably those of Raymond Roy-Camille (pedicle screws), K. Kaneda and K. Zielke (anterior plates and screws), and Y. Cotrel and J. Dubousset (pedicle screws and plates) (34,35). That said, however, and despite the advances in surgical instruments and techniques, the argument that began with Cooper and Bell is not completely resolved because patients managed without surgery can also have favorable outcomes (36–39).
Figure 8. Paul Harrington (1911–1980) introduced a system of distraction and compression rods and hooks.

Clearly, it took the accomplishments of many people from diverse fields to bring us to the point where people with SCI could be kept alive and treated operatively or nonoperatively with the prospect of living past the period of the acute injury with an aligned and stable spine. At least, the injury to the spinal column could be treated. The injury to the spinal cord itself, as well as the organ systems to which it brings innervations, have continued to pose great challenges.
Some challenges to the latter have been met. Those of the former continue to elude us. Nevertheless, as we shall see, progress in the form of treatment of the effects of SCI on the human body has been made by many who have given their careers to SCI. I think at least 6 people deserve special recognition for their contributions. I call them “heroes of the 20th century.”
Donald Munro (1898–1978) (Figure 9) has been called by some the “father of paraplegia” (40,41). Although mortality from SCI was still virtually certain (except for very incomplete injuries), Munro was unique among physicians in his day given his interest and compassion for patients with SCI and his refusal to accept the defeatist attitude toward them so widely prevalent and articulated by Cushing. He established the first spinal cord unit in the US at the Boston City Hospital in 1936 (40). He soon realized that he had to be more than just a neurosurgeon to his patients. He had to accept responsibility to provide for the whole person, who had problems involving multiple organ systems, eg, neurological, urological, orthopedic, psychological, and social. In addition, he had to coordinate all the rehabilitation efforts to improve self-care, mobility, and reassimilation into society, including educational and vocational pursuits. Although his vision was not appreciated by most of the other specialists of his day, he was successful in influencing the US army to establish SCI centers at a few hospitals (initially at Oxford-Wingate in Massachusetts), where his methods were implemented, eg, the use of “tidal drainage” to prevent recurrent urinary tract infection (UTI) (40,41). His success in the treatment of “an ailment not to be treated” served as a model for others who followed.
Figure 9. Left. Donald Munro (1898–1978) has been called the “father of paraplegia.” Photo courtesy of the Society of Neurological Surgeons. Right. Sir Ludwig Guttmann (1899–1980) was put in charge of an SCI unit at Stoke-Mandeville Hospital in 1946. Like Munro, he realized that doctors had to be interested in all the needs of the patient, not only those within one's specialty. Photo courtesy of International Spinal Cord Society, Aylesburg, Bucks, UK.

Sir Ludwig Guttmann (1899–1980) (Figure 9) is the most internationally recognized hero of this group. Born in Silesia, a long-troubled part of the globe contested for possession by Germany and Poland, he trained in Breslau (now Wroclaw) as a neurosurgeon under Otfrid Foerster (1873–1941). His practice and reputation were curtailed by the Nazis. Since he was only allowed to treat other Jews, he became chief of neurosurgery at The Breslau Jewish Hospital. He escaped from Germany to England in 1939, and in 1944 he was placed in charge of an SCI unit at Stoke-Mandeville Hospital. The care of patients with “ailments not to be treated” was never his aim in life, but fortunately, Sir Ludwig was a man who never backed away from a challenge. In learning as much as he could about SCI, he drew from the work of Munro and like the latter, he soon realized doctors treating this illness had to be rehabilitationists with a commitment to all the needs of the patient, not just those within the ambit of one's specialty (42).
Like Munro's, his SCI unit became a model for future centers. Great Britain and the rest of the British Commonwealth, Europe, and Asia all modeled their centers after Stoke-Mandeville. Unlike Munro, he strongly believed in proselytizing his experiences and traveled widely throughout the globe. A great believer in wheelchair sports, he is remembered for founding the Paralympics (42,43).
Australia was one of the places in the British Commonwealth visited by Guttmann and it was here that he met a like-minded individual who never refused a challenge and was willing to commit to SCI, namely Sir George Bedbrook (1921–1991) (Figure 10). Like Munro and Guttmann, Bedbrook soon realized he had to be a rehabilitationist, not just an orthopedic surgeon. He accepted the task of forming a spinal unit in Perth, Western Australia, and after a specially designed unit was completed in 1954, he attracted many talented people who focused their energies on studying and treating SCI, including John Pearman, microbiologist, and Byron Kakulas, neuropathologist (44).
Figure 10. Sir George Bedbrook (1921–1991) (standing) was an orthopedic surgeon who formed a spinal unit in Perth, Western Australia. He is pictured here with Sir Ludwig Guttmann (seated). Bedbrook was a dynamic visionary who traveled widely and influenced physicians, nurses, and therapists who worked with SCI patients. This photo was a gift to the author from Byron Kakulas from the Royal Perth Hospital in Perth, Western Australia.
Like Guttmann, he traveled widely, supporting the efforts of physicians starting units in Australia, New Zealand, and Africa and throughout Asia. The efforts of this dynamic visionary influenced the physicians, therapists, nurses, and all who encountered SCI patients in those parts of the world. I consider myself very fortunate to have worked with him for more than 2 years (9).
Ernest H. J. Bors (1900–1990) and A. Estin Comarr (1915–1996) (Figure 11) are usually considered together because of their numerous contributions to the field of SCI and their tireless work to provide comprehensive care at both the Long Beach, California Veterans Administration (VA) Hospital and Rancho Los Amigos Hospital in Downey, California. Their numerous articles on the neurology, especially the neurourology, of SCI remain classic. Bors was born and trained as a urologist in Prague. Like Guttmann, he was a Jewish refugee. He came to the United States and joined the Army Medical Corps. Also, like Guttmann, he established comprehensive care for a large number of patients (45,46). Comarr founded the American Paraplegia Society (APS) in 1954 (41).
Figure 11. Ernest H. J. Bors (1900–1990) (left) and A. Estin Comarr (1915–1996) (right) both established comprehensive care for a large number of patients with SCI. Courtesy of the American Paraplegia Society.
Despite the efforts of Munro, Bors, and Comarr, approaches to treatment of SCI, with some exceptions, remained fragmented, and comprehensive rehabilitation for SCI failed to become widely adopted in the Western Hemisphere until John Young (1919–1990) (Figure 12) resolved to correct this. Young was greatly influenced by the work of Guttmann and the directors of European SCI units and was an active member in what was then the International Medical Society of Paraplegia (now the International Spinal Cord Society), which was founded by Guttmann in 1961. With the assistance of J. Paul Thomas, then director of the Medical Sciences Program at the National Institute on Disability and Rehabilitation Research (NIDRR) (Figure 13), he obtained a federal grant in 1971 to demonstrate the superiority of comprehensive over fragmented SCI care in Phoenix, Arizona. He called this demonstration a “Model System.” His accomplishments were quickly recognized, and more locations were soon designated by the NIDRR as Model Systems. These Model Systems have remained in existence over the decades; they now number 14 across the US and contain the largest known database on SCI, the National Spinal Cord Injury Statistical Center database (NSCISC) (47).
Figure 12. John Young (1919–1990) (left), with the assistance of J. Paul Thomas (not shown), obtained a federal grant to demonstrate the superiority of comprehensive over fragmented SCI care. Photo courtesy of Craig Hospital Archives. Alain Rossier (right) (1930–2006) was from Switzerland but worked for 11 years in the US. He was a powerful advocate for veterans with SCI. Photo courtesy of the Swiss Paraplegic Association.
John Young stated that a “Model System must be able to meet the needs of a person with SCI by competently treating the direct injury as well as all organ systems affected (of which there are many); the functional deficits that result, by providing training and equipment; the psychological adjustments that must be made; the vocational/avocational pursuits that must be changed; and the providing of long-term specialized care.” He also outlined the necessary components of such a system, which must include emergency medical services; emergency trauma care (at a trauma center); acute hospital care; acute rehabilitation care; and ongoing rehabilitation treatment (47,48).
Finally, Alain Rossier (1930–2006) (Figure 12), born in Switzerland, was strongly influenced by Guttmann and was also influential in establishing SCI units in his country. He also spent 11 years working in the United States at the West Roxbury VA Hospital, MA (49). He proved to be a powerful advocate for veterans with SCI and convinced the VA that more VA hospitals should have SCI units and that these units should be equipped to meet all their patients' medical and social needs throughout their lives.
Consequently, to this day, patients with SCI requiring readmission to the designated VA hospitals are admitted to the SCI unit regardless of whether their status is acute or chronic (unless they require intensive care). Today, this stands in contrast to most health care payers, who authorize payments to SCI centers for acute rehabilitation or for rehabilitation related to chronic issues that can be resolved with a short-term admission. More and more non-VA patients with acute medical problems, eg, acute UTI, are admitted to acute general hospitals, while patients with chronic medical problems, eg, decubitus ulcers, are admitted to long-term care facilities or are treated at home. This system exposes the patient to fragmentation of care and to the risk of complications, as the staffs in these settings are less acquainted with the special needs of patients with SCI. In settings where these conditions prevail, it is important that the rehabilitationist bring the principles developed by these heroes and others to the patient. The present challenge is, how?
IN THE PRESENT—AN AILMENT TO BE TREATED
If one were to ask where the work of these heroes as well as that of many others (eg, Key of South Africa, Botterell of Canada, Holdsworth of the UK, Meineke of Germany, Dolfus of France, Chahal of India, Nakamura of Japan, Sarias of Spain, and Stover of the US, to mention a few) have brought us today, we could point to the advances made in medical care; longevity; rehabilitation services, including mobility and self-care; environmental adaptations and legislation; and the numerous organizations pledged to mainstream the lives of persons with SCI and advance the search for a cure. A brief sampling of these is mentioned here.
Medical Care
The Spine
As noted, instruments and techniques now exist to assure spinal stability, obtain reductions, maintain alignment, and thereby avoid the pain and further disability caused by a deformed spine (9). In addition, imaging capabilities have expanded significantly, not only with respect to the ability to correlate MRI with impairment (50,51) and prognosis (52,53), but with new MRI applications such as magnetic resonance imaging–diffusion weighted imaging (54,55), which allows imaging of the tracts within the spinal cord; functional MRI (56,57); and MRI spectroscopy (58,59).
The Neurogenic Bladder
Advances in this area are the reason why pyelonephritis is no longer the “end condition of the paraplegic.” These include antibiotics to treat and prevent infection of the genitourinary (GU) tract; anticholinergic medications to maintain continence and bladder compliance; less invasive ways to remove bladder and kidney stones, eg, electrohydraulic lithotripsy (EHL) and extracorporeal shock wave lithotripsy (ESWL); urodynamics to help us unravel the mystery of the neurogenic bladder and recommend the best treatment approaches; and surgical procedures to enhance bladder storage capacity, facilitate bladder emptying, or increase the ease of catheterization. These and other treatments have enhanced social continence and quality of life and have prevented many of the complications that previously shortened life (60–65).
Sexuality
Men with SCI have benefited from the development of oral agents to treat erectile dysfunction, eg, sildenafil citrate, as well as the injectable prostaglandin E1 (alprostadil) for those who do not respond to oral therapy. Since many men with SCI also have ejaculatory dysfunction, their ability to father a child by means of vibratory or rectal electric probe ejaculation, combined with in-vitro fertilization, has been greatly enhanced so that fatherhood is now commonplace. Women with SCI have also benefited, particularly from safer labor and delivery and if necessary, safer cesarean sections due to advances in surgery mentioned earlier. Both men and women have the opportunity for more satisfying sexual relations (65–70).
Pain
Neuropathic pain has long been a problem for many persons with SCI (50). The discovery that certain anticonvulsant and antidepressant medications can suppress or relieve pain perceived by the patient either at the level of injury or diffusely below it has improved the quality of life for many. Invasive procedures such as implantable spinal cord stimulators and the infusion of morphine and clonidine intrathecally have proved helpful in selected cases (71–76).
Spasticity
Involuntary movement and involuntary resistance to passive movement occur typically in persons with SCI above the conus level. These movements can become very strong and have impeded many from achieving independence in activities of daily living. Oral agents such as baclofen, tizanidine, and dantrolene have been helpful in reducing spasticity (77). However, the arrival of intrathecal baclofen, which is used to treat those who do not respond sufficiently to oral medication, has proved to be a breakthrough (78,79). Botulinum toxin, when injected into a few offending muscles, has also been very helpful (80,81).
Advances in many other areas important to individuals with SCI, including the skin, bowel, bone, lung, and cardiovascular system, have also been made but are too numerous to mention here. Suffice it to say, people can be kept healthy and more comfortable because of them. All this has fostered their re-entry into society.
Longevity
In years past, as noted above, the leading cause of death among persons with SCI was renal failure. Today, however, significant advances in urologic management have resulted in dramatic shifts in the leading causes of death. Persons enrolled in the National SCI Statistical Center Database since its inception in 1973 have now been followed for more than 30 years after injury. During that time, the causes of death that appear to have the greatest impact on reduced life expectancy for this population have changed and are now pneumonia, pulmonary emboli, and septicemia (84,85). More importantly, people with SCI are living longer (84).
Rehabilitation Services
In addition to the expert clinical, hands-on treatment now available to individuals with SCI both during acute rehabilitation and during ongoing outpatient care, especially in spinal centers, they can benefit from advances in technology that have yielded better equipment.
Power wheelchairs can now perform weight-shifting functions by tilting and/or reclining. They can be controlled by mechanisms other than the joystick, eg, head control, sip and puff, and voice and eye movement. This has helped individuals with high tetraplegia expand their mobility with less stress on the shoulder joints (86,87).
Manual wheelchairs are now lighter, making them easier to propel and load into a vehicle. They can also be equipped with power-assist wheels, allowing people with less upper body strength to push farther (88).
Lightweight orthotic devices for both upper and lower extremities have made donning and doffing easier and decreased energy cost for ambulatory persons (89,90).
Computer interfaces have allowed individuals with high tetraplegia to access the Internet and use other computer applications, permitting them to experience the benefits (and the caveats) just like anyone else (91,92).
Pressure mapping to help select the best wheelchair cushion has proven useful to all wheelchair users but especially to individuals more vulnerable to pressure ulcers, ie, those who cannot perform a manual weight shift or who have severe gluteal muscular atrophy or scars on the weight-bearing surface (93,94).
Body weight–supported treadmill ambulation has both research and clinical applications. Although research is still ongoing on this equipment, it is now proving clinically useful as a training device for persons with marginal ambulatory capability. It has thus far been found useful as an adjunct to the treatment of patients with some preserved motor function (ASIA Impairment Scales C and D) (95).
Other technological advances have improved the self-care, mobility, and independence of persons with SCI; they include functional electrical stimulation (89), speaking valves for tracheostomies, and environmental control systems (96).
Environmental Adaptations/Legislation
Environmental Control Systems
What is sophisticated and extremely useful for those who can afford it is the ability to control devices within the home from a remote source, eg, wheelchair or bedroom. This might include the climate control panel, radio/TV/CD player, alarm systems, door locks, and other vital functions within the person's home (96).
Home Modifications
Centers providing comprehensive rehabilitation have planned their discharges from acute rehabilitation at the point when the patient's abilities matched his/her home environment. Usually, the environment has to be modified to effect this match. This often includes the installation of ramps; widening of doorways; altering the bathroom, kitchen, and bedroom; and so forth. There is usually nothing sophisticated about the implementation of these changes. The challenge is often getting insurers to pay for them.
Legislation
The Americans with Disabilities Act signed by President George H. W. Bush in 1990 was a landmark piece of legislation that declared that Americans with disabilities have equal rights to the environment, employment (if otherwise qualified), public transportation, accommodations, services, and education. By all accounts, it was a major breakthrough for persons with SCI, as well as those with other disabilities (97).
Organizations
Many consumer groups have formed throughout the nation and the world to promote the interests of individuals with SCI. They run the gamut in their mission statements. Some are primarily social, some political, some educational, some philanthropic, eg, funding research, while others have combinations of these purposes. One cannot list all of them here but 3 certainly stand out because of their size: The National Spinal Cord Injury Association, Paralyzed Veterans of America, and United Spinal Association.
Similarly, many professional groups have formed, usually declaring educational and/or scientific goals for their organizations. The International Spinal Cord Society (ISCoS) changes its venues yearly, routinely combining its annual scientific meeting with that of the national SCI organization of the host country. In the US, the American Spinal Injury Association (ASIA) and the American Paraplegia Society (APS) each have annual meetings. ASIA and ISCoS have jointly produced the International Standards for Neurological Classification of SCI. This has now become the gold standard clinical measurement tool used for outcome measurement in SCI research on humans (98).
Further, many organizations have formed with at least one of their objectives being the finding of a way to cure or reverse the damage caused by injury or other pathologic processes to the spinal cord. One of them, the International Campaign for Cures of Spinal Cord Injury Paralysis (ICCP) has at present 9 member organizations: The Christopher Reeve Paralysis Foundation, The Miami Project to Cure Paralysis, The Paralyzed Veterans of America, the French Institute for Spinal Cord Research, The Japan Spinal Cord Foundation, The Rick Hansen Man in Motion Foundation, Spinal Cure Australia, Spinal Treatment Australia, and The International Spinal Research Trust. By their names, it is clear that “cure” is their raison d'etre. Other organizations at the forefront in this effort are the International Collaboration on Repair Discoveries (ICORD), the North American Clinical Trials Network (NACTN), and the European Clinical Trials Network (EUCTN). In addition, there are many smaller groups, often university based, such as “Mission Connect,” which is funded through the efforts of the Institute for Rehabilitation and Research (TIRR) in Houston, Texas.
Governmental organizations have also played no small role in supporting research activities. These include the World Health Organization (WHO), the National Institute for Disability and Rehabilitation Research (NIDRR), the National Institutes of Health, especially the National Center for Medical Rehabilitation and Research (NCMRR), and the National Institute for Neurological Diseases and Stroke (NINDS). Likewise, as we shall see, the pharmaceutical industry is conducting research investigations including clinical trials.
All the resources of these groups are working toward bringing us to the day when we can declare a cure for SCI.
IN THE FUTURE—AN AILMENT TO BE CURED
The following organizations are planning for the sound implementation of clinical trials as interventions uncovered by basic science researchers come on line. The ICCP has taken the initiative by producing 4 reports thus far (99). At the first meeting, topics discussed included the prevalence and incidence of SCI, both tetraplegia and paraplegia, in countries where they are known; the natural history of recovery of neurologic function from information contained in the NSCISC Database and other sources; and the statistical power required to conduct clinical trials. At the second meeting, outcome measures appropriate to studies of acute, subacute, and chronic SCI were discussed, along with the important differences between parameters that measure axonal connectivity and those that measure function. It is important not to confuse or commingle the two. At the third meeting, inclusions and exclusion criteria were addressed, along with the ethics of how they affect control groups. Finally, the fourth meeting reviewed the overall principles involving clinical trials and how they pertain to SCI, emphasizing randomized prospective clinical trials (RPCT), multicenter trials (due to the relatively low incidence of SCI), the importance of blinding, and the importance of the adherence to the ethical principles of the Declaration of Helsinki (100) and the Belmont Report (101). This effort has laid the groundwork for multicenter and multinational collaboration in the conduct of clinical trials within the ambit of NACTN and EUCTN (102).
Clinical trials are in fact underway; many utilizing new discoveries developed by the pharmaceutical industry in Europe, North America, and Asia (103). These include: (a) the Proneuron Phase II trial using autologous incubated macrophages for acute SCI (104); (b) the BioAxone Cethrin trial using the Rho antagonist BA-210 (Cethrin) for acute SCI (105); (c) the University of Calgary trial, using minocycline (an antibiotic with cytokine, free radical, and matrix metalloproteinase inhibition properties) for acute SCI (106); (d) the Aventis HP-184 trial employing this substance in chronic SCI in which some motor function has been preserved—this substance is similar to 4-amino pyridine (4-AP); (e) the Novartis trial using the anti-NoGo antibody, which reverses the inhibiting effect of NoGo on oligodendrocytes for acute SCI (107); (f) the Geron Corporation trial using implanted oligodendrocyte precursor cells for chronic SCI (108,109); (g) transplantation of fetal and autologous olfactory cells into the spinal cord for chronic SCI in trials in Portugal and China (110,111).
Clinical trials already completed include those studying the effects of methyl-prednisolone (10,112–114), GM-1 ganglioside (11,115), 4-AP (116–118), and body weight–supported treadmill ambulation (95). Thus far, in sum, these studies have proved inconclusive.
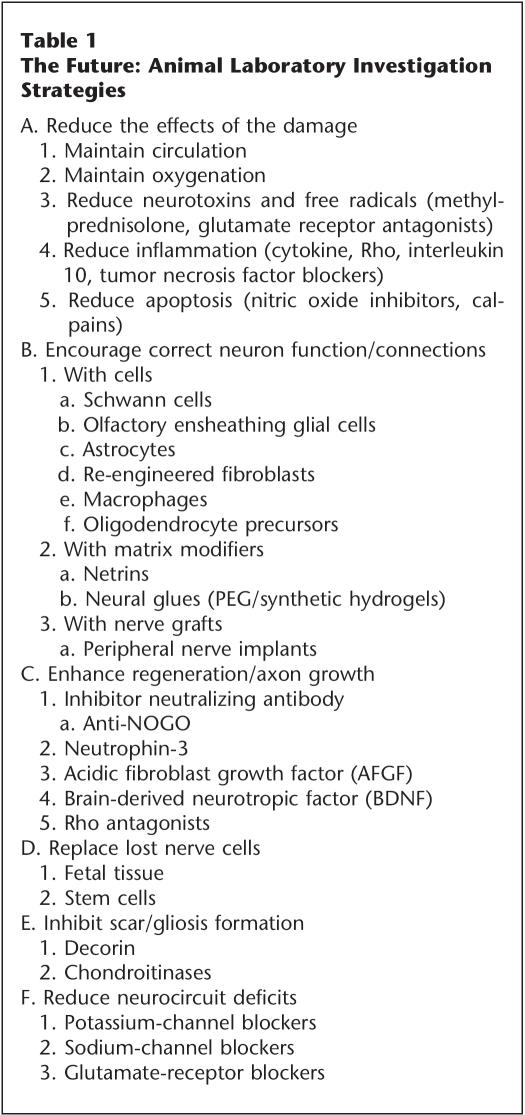
Research scientists at the animal laboratory level have developed multiple strategies for studying and repairing the injured spinal cord including both acute and chronic subjects (Table 1). These efforts have been quietly taking place for more than a decade and have evolved into numerous investigations approaching the problem from different angles. These strategies include:
Table 1.
The Future: Animal Laboratory Investigation Strategies
A. Reduce the Effects of the Damage
This can be accomplished or aided by maintaining circulation and oxygenation and creating a favorable milieu (realign and stabilize) (9). Partially damaged neural tissue may also be protected by the reduction of neurotoxins and free radicals, eg, by methylprednisolone or glutamate-receptor (eg, AMPA and NMDA) antagonists (119,120); the reduction of inflammation, eg, by cytokine, Rho, tumor necrosis factor, and interleukin 10 blockers; and by the reduction of apoptosis, eg, by calpains or nitric oxide inhibitors (104,121–125).
B. Encourage Correct Neuron Function/Connections Via a Nerve Bridge
This can be done in 3 ways: 1. With Cells: Schwann cells (126–128); olfactory ensheathing glial cells (125,126,128,129); astrocytes (131); re-engineered fibroblasts or other cell types (132,133). 2. With Matrix Modifiers: These include netrins (134) and neural glues, eg, PEG/synthetic hydrogels (135–137). 3. With Nerve Grafts: ie, peripheral nerve implants into the spinal cord after removal of glial remnants (137).
C. Enhance Regeneration/Axon Growth
Several interesting approaches have shown promise in animal studies. These include inhibitor-neutralizing antibody (anti-NoGo) (138); neurotrophin 3 (139); acidic fibroblast growth factor (AFGF) (139); brain-derived neurotrophic factor (BDNF) (140); and Rho antagonists (105).
D. Replace Lost Nerve Cells
Currently, fetal tissue implants (141, 142) and stem cells (143) are being studied.
E. Inhibit Scar/Gliosis Formation
Both decorin (144) and chondroitinases (126,145) have shown promise in work done thus far.
F. Reduce Neurocircuit Deficits
Both potassium-channel blockers (118,121) and sodium-channel blockers (121) are being studied for this purpose, along with glutamate-receptor blockers (119).
CONCLUSION
It is both amazing and gratifying to see the enormous amount of work now being done to solve the mystery of spinal cord regeneration. Most investigators feel the solution to the enigma will come from this multipronged approach, since very likely genes, molecules, and milieu all play some role (146). With perseverance by the scientists and support from the consumers, like that given by the late and heroic Christopher Reeve, someday, in the great beyond, even though he lived 5,000 years afterwards, the latter might just confront Imhotep and say, “See, it can be treated and cured after all.”
Footnotes
Based on the author's Donald Munro Lecture presented at the 52nd annual meeting of the American Paraplegia Society in Las Vegas, Nevada, September 2006.
REFERENCES
- Santayana G. The Life of Reason, Reason in Common Sense. New York: Charles Scribner's; 1905. p. 284. [Google Scholar]
- Hughes JT. The Edwin Smith Papyrus; an analysis of the first case reports of spinal cord injuries. Paraplegia. 1988;26:71–82. doi: 10.1038/sc.1988.15. [DOI] [PubMed] [Google Scholar]
- Breasted JH. Edwin Smith Surgical Papyrus in Facsimile and Hieroglyphic Transliteration With Translation and Commentary. 2 Vols. Chicago, IL: University of Chicago Oriental Institute Publications; 1930. [Google Scholar]
- Guttmann L. Spinal Cord Injuries. Comprehensive Management and Research. London, England: Blackwell Scientific Publications; 1976. pp. 4–7. [Google Scholar]
- Wang D, El-Masry WS, Crumplin M, Eisenstein S, Pusey RJ, Meagher T. Admiral Lord Nelson's death: known and unknown—a historical review of the anatomy. Spinal Cord. 2005;43:573–576. doi: 10.1038/sj.sc.3101850. [DOI] [PubMed] [Google Scholar]
- Eltorai I, Garfield JA. J Spinal Cord Med. 2004. pp. 330–341. [DOI] [PubMed]
- Silver JR. History of the Treatment of Spinal Injuries. London, England: Kluwer Academic/Plenum Publishers; 2005. pp. 11–84. [Google Scholar]
- Essame H. Patton as Military Commander. Conshohocken, PA: Combined Publishing; 1973. [Google Scholar]
- Donovan WH. Operative and non-operative management of spinal cord injury: a review. Paraplegia. 1994;32:375–388. doi: 10.1038/sc.1994.64. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shephard MJ, Collins WH, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury: result of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Dorsay FE, Coleman WP. Recovery of motor function after spinal cord injury—a randomized placebo controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- Hart M. The 100 Most Influential Persons of History. New York, NY: Hart Publishing Co; 1978. p. 95. [Google Scholar]
- Wiltse LL. In: The History of Spinal Disorders in The Adult Spine: Principles and Practice. Frymoyer JW, editor. New York: Raven Press Ltd; 1991. pp. 3–16. [Google Scholar]
- Lister J. Antiseptic principles in the practice of surgery. BMJ. 1867;2:9–12. doi: 10.1136/bmj.2.5543.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. J Barth. Leipzig: 1912. Die actiolage Dr. Mitzbrand-Krankheit Klassker der Medizen. [Google Scholar]
- Thurwood J. The Century of the Surgeon. New York, NY: Pantheon Books; 1957. pp. 280–289.pp. 296–301. [Google Scholar]
- McDermott W, Rogers DE. Social ramifications of control of microbial disease. John Hopkins Med J. 1982;151:301–312. [PubMed] [Google Scholar]
- Gowlland EL. The after treatment of paraplegia following spinal injuries and of disseminated sclerosis. Medical Press Circular. 1934. pp. 81–84.
- Morton WTG. A memoir to The Academy of Sciences at Paris on a new use of sulfuric ether. Little's Living Age. 1848;16:529–571. [Google Scholar]
- Kitz RJ, Vandam LD. A history and the scope of anesthetic practice. In: Miller RD, editor. Anesthesia. Vol 1. London, England: Churchill Livingstone; 1986. pp. 3–12. [Google Scholar]
- Wintrobe MM. The Blood Groups and Blood Transfusion in Clinical Hematology. Philadelphia, PA: Lea & Febiger; 1962. pp. 338–375. [Google Scholar]
- Wiltse LL. In: The History of Spinal Disorders in The Adult Spine: Principles and Practice. Frymoyer JW, editor. New York: Raven Press Ltd; 1991. pp. 3–16. [Google Scholar]
- Sicard JA, Forestiere J. Methode radiographique d'exploration de la cavité epidurale par le lipiodol. Rev Neurol. 1921;37:1264–1266. [Google Scholar]
- Houndsfield G. The Quest for an Image of the Brain. New York, NY: Raven Press; 1980. Quoted by Oldendorf WH. [Google Scholar]
- Moore H. The Quest for an Image of the Brain. New York, NY: Raven Press; 1980. Quoted by Oldendorf WH. [Google Scholar]
- Reid A, Smith FW, Hutchinson JM. Nuclear magnetic resonance and its safety implications: follow-up of 181 patients. Br J Radiol. 1982;55:784–786. doi: 10.1259/0007-1285-55-658-784. [DOI] [PubMed] [Google Scholar]
- Richards DW. Hippocrates of Ostia. JAMA. 1968;204:1049–1056. [PubMed] [Google Scholar]
- Jefferson G. The treatment of spinal injuries. Practitioner. 1933;130:332–341. [Google Scholar]
- Crutchfield WG. Skeletal traction for dislocation of the cervical spine—report of a case. Southern Surgeon. 1933;2:156–159. [Google Scholar]
- Crutchfield WG. Treatment of injuries of the cervical spine. J Bone Joint Surg. 1938;20:696–704. [Google Scholar]
- Nickel VL, Perry J, Garrett A, et al. The halo. A spinal skeletal traction fixation device. J Bone Joint Surg Am. 1968;50a:1400–1409. [PubMed] [Google Scholar]
- Harrington PR. Treatment of scoliosis. J Bone Joint Surg Am. 1962;44:591–610. [PubMed] [Google Scholar]
- Katznelson AM. Stabilization of the spine in traumatic paraplegia. Paraplegia. 1969;7:33–37. doi: 10.1038/sc.1969.8. [DOI] [PubMed] [Google Scholar]
- Roy-Camille R, Saillant G, Magel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop. 1986;203:7–17. [PubMed] [Google Scholar]
- Meyer P., Jr . Surgery of Spinal Trauma. New York, NY: Churchill Livingstone; 1989. [Google Scholar]
- El Masry WS, Osman AE. Management of the Spinal Injury. In: Cassar-Pullicino VN, editor. Clinical Perspectives on Spinal Injuries. Spinal Trauma — An Imaging Approach. Stuttgart, Germany: Gerog Thieme Verlag; 2006. pp. 9–11. [Google Scholar]
- Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36:S-B13–S-B26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- El Masri(y) WS, Katoh S, Khan A. Reflections on the neurological significance of bony canal encroachment following traumatic injury of the spine in patients with Frankel C D and E presentation. J Neurotrauma. 1993;10(Suppl):70. [Google Scholar]
- El Masri(y) WS, Meerkotter DV. Spinal cord dysfunction. Intervention & treatment. In: Illis LS, editor. Early Decompression of the Spinal Cord Following Injury: Arguments for and Against. Vol II. New York, NY: Oxford University Press; 1992. pp. 7–27. [Google Scholar]
- Silver JR. History of the Treatment of Spinal Injuries. London, England: Kluwer Academic/Plenum Publishers; 2005. pp. 111–119. [Google Scholar]
- Eltorai IM. History of spinal cord medicine. In: Lin V, editor. Spinal Cord Medicine, Principles and Practice. New York, NY: Demos Publications; 2002. pp. 3–14. [Google Scholar]
- Guttmann L. Management of Spinal Fractions. In: Guttmann L, editor. Spinal Cord Injuries, Comprehensive Management and Research. London: Blackwell Scientific Publications, Oxford Press; 1976. pp. 7–21. [Google Scholar]
- Silver JR. History of the Treatment of Spinal Injuries. London, England: Kluwer Academic/Plenum Publishers; 2005. pp. 69–98. [Google Scholar]
- Bedbrook GM. The Lifetime Care of the Paraplegic Patient. Melbourne, Australia: Churchill Livingstone; 1985. [Google Scholar]
- Silver JR. History of the Treatment of Spinal Injuries. London, England: Kluwer Academic/Plenum Publishers; 2005. pp. 123–125. [Google Scholar]
- Bors E. The Spinal Cord Injury Center of the Veterans Administration Hospital, Long Beach, California, USA. Paraplegia. 1967;5:126–130. doi: 10.1038/sc.1967.15. [DOI] [PubMed] [Google Scholar]
- Stover SL, DeVivo MJ, Go BK. History, implementation and current status of the National Spinal Cord Injury Database. Arch Phys Med Rehabil. 1999;80:1365–1371. doi: 10.1016/s0003-9993(99)90246-0. [DOI] [PubMed] [Google Scholar]
- Thomas JP. Rehabilitation of the spinal cord injured: the model systems approach. SCI Digest. 1970. pp. 3–6.
- Tricot A. Professor Alain B. Rossier, MD, Immediate past president and medallist of the International Medical Society of Paraplegia: A profile. Paraplegia. 1989;27:81–84. doi: 10.1038/sc.1989.13. [DOI] [PubMed] [Google Scholar]
- Marciello MA, Flanders AE, Herbison GJ, Schafer DM, Friedman DP, Lane J, et al. Magnetic resonance imaging related to neurologic outcome in cervical spinal cord injury. Arch Phys Med Rehabil. 1993;74:940–946. [PubMed] [Google Scholar]
- Selden N, Quint D, Patel N, et al. Emergency magnetic resonance imaging of cervical spinal cord injuries: clinical correlation and prognosis. Neurosurgery. 1999;44:785–792. doi: 10.1097/00006123-199904000-00057. [DOI] [PubMed] [Google Scholar]
- Flanders A, Spettell C, Tartaglino L, et al. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–655. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- Flanders A, Spettel C, Friedman D, et al. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. Am J Neuroradiol. 1999;20:926–934. [PMC free article] [PubMed] [Google Scholar]
- Schwartz E, Chin C, Shumsky J, et al. Apparent diffusion coefficients in spinal cord transplants and surrounding white matter correlate with degree of axonal dieback after injury in rats. Am J Neuroradiol. 2005;26:7–18. [PMC free article] [PubMed] [Google Scholar]
- Schwartz E, Hackney D. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol. 2003;184:570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Nevo U, Hauben E, Yoles E, Agranov E, et al. Diffusion anisotropy MRI for quantitative assessment of recovery in injured rat spinal cord. Magn Reson Med. 2001;45:1–9. doi: 10.1002/1522-2594(200101)45:1<1::aid-mrm1001>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Curt A, Alkadhi H, Crelier G, et al. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain. 2002;125:2567–2578. doi: 10.1093/brain/awf250. [DOI] [PubMed] [Google Scholar]
- Pattany P, Yezierski R, Wilderstrom-Noga E, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- Puri B, Smith H, Cox I, et al. The human motor cortex after incomplete spinal cord injury: an investigation using proton magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1998;65:748–754. doi: 10.1136/jnnp.65.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binard JE, Persky L, Lockhart JL, Kelley B. Intermittent catheterization the right way! (volume vs. time-directed) J Spinal Cord Med. 1996;19:194–196. doi: 10.1080/10790268.1996.11719432. [DOI] [PubMed] [Google Scholar]
- Chai T, Chung AK, Belville WD, Faerber GJ. Compliance and complications of clean intermittent catheterization in the spinal cord injured patient. Paraplegia. 1995;33:161–163. doi: 10.1038/sc.1995.35. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F. Urinary tract infection in individuals with spinal cord lesion. Curr Opin Urol. 2002;12:45–49. doi: 10.1097/00042307-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Shin JC, Park CI, Kim HJ, Lee IY. Significance of low compliance bladder in cauda equine injury. Spinal Cord. 2002;40:650–655. doi: 10.1038/sj.sc.3101380. [DOI] [PubMed] [Google Scholar]
- Husmann OA, Cain MP. Fecal and urinary continence after ileal cecal cystoplasty for the neurogenic bladder. J Urol. 2001;165:922–925. [PubMed] [Google Scholar]
- Nomura S, Ishido T, Tanaka K, Komiya A. Augmentation ileocystoplasty in patients with neurogenic bladder due to spinal cord injury or spina bifida. Spinal Cord. 2002;40:30–33. doi: 10.1038/sj.sc.3101249. [DOI] [PubMed] [Google Scholar]
- Courtois FJ, Charvier KF, Leriche A, Raymond DP, Eyssette M. Clinical approach to erectile dysfunction in spinal cord injured men. A review of clinical and experimental data. Paraplegia. 1995;33:628–635. doi: 10.1038/sc.1995.133. [DOI] [PubMed] [Google Scholar]
- Schmid DM, Schurch B, Hauri D. Sildenafil in the treatment of sexual dysfunction in spinal cord-injured male patients. Eur Urol. 2000;38:184–193. doi: 10.1159/000020278. [DOI] [PubMed] [Google Scholar]
- Smith EM, Bodner DR. Sexual dysfunction after spinal cord injury. Urol Clin North Am. 1993;20:535–542. [PubMed] [Google Scholar]
- Kreuter M, Sullivan M, Siosteen A. Sexual adjustment and quality of relationship in spinal paraplegia: a controlled study. Arch Phys Med Rehabil. 1996;77:541–548. doi: 10.1016/s0003-9993(96)90292-0. [DOI] [PubMed] [Google Scholar]
- Sipski ML, Alexander CJ. Sexual activities, response and satisfaction in women pre- and post-spinal cord injury. Arch Phys Med Rehabil. 1993;74:1025–1029. doi: 10.1016/0003-9993(93)90056-g. [DOI] [PubMed] [Google Scholar]
- Rutkowski SB, Middleton JW, Truman G, Hagen DL, Ryan JP. The influence of bladder management on fertility in spinal cord injured males. Paraplegia. 1995;33:263–266. doi: 10.1038/sc.1995.59. [DOI] [PubMed] [Google Scholar]
- Siddall P, McClelland J, Rutkowski S, et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, De Lisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. 2002;25:100–105. doi: 10.1080/10790268.2002.11753609. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Molloy AR, Walker S, Mather LE, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493–1498. doi: 10.1097/00000539-200012000-00037. [DOI] [PubMed] [Google Scholar]
- Krames E. Implantable devices for pain control: spinal cord stimulation and intrathecal therapies. Best Pract Res Clin Anaesthesiol. 2002;16:619–649. doi: 10.1053/bean.2002.0263. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Middleton JW. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006;44:67–77. doi: 10.1038/sj.sc.3101824. [DOI] [PubMed] [Google Scholar]
- Taricco M, Adone R, Pagliacci C, Telaro E. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;2:CD001131. doi: 10.1002/14651858.CD001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenkov AI, Niendorf WR, Darwish N, Glaeser E, Gaab MR. Continuous intrathecal infusion of baclofen in patients with spasticity caused by spinal cord injuries. Neurosurg Rev. 2002;25:228–230. doi: 10.1007/s10143-002-0221-1. [DOI] [PubMed] [Google Scholar]
- Lewis KS, Mueller WM. Intrathecal baclofen for severe spasticity secondary to spinal cord injury. Ann Pharmacother. 1993;27:767–774. doi: 10.1177/106002809302700618. [DOI] [PubMed] [Google Scholar]
- Al-Khodairy AT, Gobelet C, Rossier AB. Has botulinum toxin type A a place in the treatment of spasticity in spinal cord injury patients? Spinal Cord. 1998;36:854–858. doi: 10.1038/sj.sc.3100703. [DOI] [PubMed] [Google Scholar]
- Adams MM, Hicks AL. Spasticity after spinal cord injury. Rev Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- DeVivo M, Go B. Facts and Figures at a Glance. Birmingham, AL: National Spinal Cord Injury Statistical Center; 2005. [Google Scholar]
- DeVivo M, Go B, Jackson A. Overview of the National Spinal Cord Injury Statistical Center Database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Levy CE, Bonninger ML, McDermott M, Berner TF. Wheeled mobility. In: Grabois M, Garrison SJ, Hart KA, Lehmkuhl LD, editors. Physical Medicine and Rehabilitation: The Complete Approach. Malden, MA: Blackwell Science, Inc; 2000. pp. 685–711. [Google Scholar]
- Kirshblum S. New rehabilitation interventions in spinal cord injury. J Spinal Cord Med. 2004;27:342–350. doi: 10.1080/10790268.2004.11753772. [DOI] [PubMed] [Google Scholar]
- Algood SD, Cooper RA, Fitzgerald SG, Cooper R, Boninger ML. Effect of a pushrim-activated power-assist wheelchair on the functional capabilities of persons with tetraplegia. Arch Phys Med Rehabil. 2005;86:380–386. doi: 10.1016/j.apmr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- To CS, Kirsh RF, Kobetic R, Triolo RJ. Simulation of a functional neuromuscular stimulation powered mechanical gait orthosis with coordinated joint locking. IEEE Trans Neural Syst Rehabil Eng. 2005;13:227–235. doi: 10.1109/TNSRE.2005.847384. [DOI] [PubMed] [Google Scholar]
- Stallard J, McLeod N, Woollam PJ, Miller K. Reciprocal walking orthosis with composite material body brace: initial development. Proc Inst Mech Eng [H] 2003;217:385–392. doi: 10.1243/095441103770802559. [DOI] [PubMed] [Google Scholar]
- Cooper RA. Bioengineering and spinal cord injury: a perspective on the state of the science. J Spinal Cord Med. 2004;27:351–364. doi: 10.1080/10790268.2004.11753773. [DOI] [PubMed] [Google Scholar]
- Birch GE, Bozorgzadeh Z, Mason SG. Initial on-line evaluations of the LF-ASD brain-computer interface with able-bodied and spinal-cord subjects using imagined voluntary motor potentials. IEEE Trans Neural Syst Rehabil Eng. 2002;10:219–224. doi: 10.1109/TNSRE.2002.806839. [DOI] [PubMed] [Google Scholar]
- Yuen HK, Garrett D. Comparison of three wheelchair cushions for effectiveness of pressure relief. Am J Occup Ther. 2001;55:470–475. doi: 10.5014/ajot.55.4.470. [DOI] [PubMed] [Google Scholar]
- Henderson JL, Price SH, Brandstater ME, Mandac BR. Efficacy of three measures to relieve pressure in seated persons with spinal cord injury. Arch Phys Med Rehabil. 1994;75:535–539. [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs. over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Tran Y, McIsaac P, Boord P. The efficacy and benefits of environmental control systems for the severely disabled. Med Sci Monit. 2005;11:RA32–39. [PubMed] [Google Scholar]
- Frieden L. Listening for footsteps. Arch Phys Med Rehabil. 2002;83:150–153. doi: 10.1053/apmr.2002.31760. [DOI] [PubMed] [Google Scholar]
- Marino R, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (6th edition) J Spinal Cord Med. 2003;26(Suppl 1):S49–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Adams M, Cavanagh JFR. International campaign for cures of spinal cord injury paralysis (ICCP): another step forward for spinal cord injury research. Spinal Cord. 2004;42:273–280. doi: 10.1038/sj.sc.3101597. [DOI] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helinski. Ethical Principles for Medical Research Involving Human Subjects. Helinski, Finland, June 1964, as amended 2004. Available at: http://www.wma.net/e/policy/b3.htm. Accessed Feb 15, 2007.
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . Washington, DC: 1979. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Available at: http://www.ohsr.od.nih.gov/guidelines/belmont.html. Accessed Feb 15, 2007. [PubMed] [Google Scholar]
- Steeves J, Fawcett J, Tuszynski M. Report of International Clinical Trials Workshop on Spinal Cord Injury February 20–21, 2004, Vancouver, Canada. Spinal Cord. 2004;42:591–597. doi: 10.1038/sj.sc.3101669. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Spinal Cord Injury: Progress, Promise and Priorities. National Academy Press; Washington, DC: 2005. [Google Scholar]
- Bomstein Y, Marder JB, Vitner K, et al. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ. Lesional RhoA+cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia. 2004;47:377–386. doi: 10.1002/glia.20031. [DOI] [PubMed] [Google Scholar]
- Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–1106. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multi-neurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers L, Maroon JC, Marshall LF, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second national acute spinal cord injury study. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow-up. Results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D, the Sygen Study Group Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine. 2001;26:S68–S86. doi: 10.1097/00007632-200112151-00014. [DOI] [PubMed] [Google Scholar]
- Donovan WH, Halter JA, Graves DE, et al. Intravenous infusion of 4-AP in chronic spinal cord injured subjects. Spinal Cord. 2000;38:7–15. doi: 10.1038/sj.sc.3100931. [DOI] [PubMed] [Google Scholar]
- Hansebout RR, Blight AR, Fawcett S, Reddy K. 4-Amino-pyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J Neurotrauma. 1993;10:19–24. doi: 10.1089/neu.1993.10.1. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Potter PJ, Wolfe DL, Hsich JT, Delaney GA, Blight AR. 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. J Neurotrauma. 1994;11:433–446. doi: 10.1089/neu.1994.11.433. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Hughes MG, Nie L, et al. The effect of glutamate receptor blockers on glutamate release following spinal cord injury. Lack of evidence for an ongoing feedback cascade of damage !glutamate release !damage !glutamate release !, etc. Brain Res. 2005;1038:92–99. doi: 10.1016/j.brainres.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Mignani S, Bohme GA, Birraux G, et al. 9-Carboxymethyl-5H, 10H-imidazo[1,2-a]indeno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg Med Chem. 2002;10:1627–1637. doi: 10.1016/s0968-0896(01)00431-x. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Yune TY, Chang MJ, Kim SJ, et al. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma. 2003;20:207–219. doi: 10.1089/08977150360547116. [DOI] [PubMed] [Google Scholar]
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Harrington JF, Messier AA, Levine A, Szmydynger-Cho-dobska J, Chodobski A. Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma. 2005;22:919–928. doi: 10.1089/neu.2005.22.919. [DOI] [PubMed] [Google Scholar]
- Kwak EK, Kim JW, Kang KS, et al. The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci. 2005;20:663–669. doi: 10.3346/jkms.2005.20.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transaction of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyvan-Fouladi N, Raisman G, Li Y. Delayed repair of corticospinal tract lesions as an assay for the effectiveness of transplantation of Schwann cells. Glia. 2005;51:306–311. doi: 10.1002/glia.20211. [DOI] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Honig WM, et al. Olfactory ensheathing cells, olfactory nerve fibroblasts and biomatrices to promote long-distance axon regrowth and functional recovery in the dorsally hemisected adult rat spinal cord. Exp Neurol. 2006;200:89–103. doi: 10.1016/j.expneurol.2006.01.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005;52:245–251. doi: 10.1002/glia.20241. [DOI] [PubMed] [Google Scholar]
- Marshall CT, Lu C, Winstead W, et al. The therapeutic potential of human olfactory-derived stem cells. Histol Histopathol. 2006;21:633–643. doi: 10.14670/HH-21.633. [DOI] [PubMed] [Google Scholar]
- Davies J, et al. 2006. Presented at the 2nd Combined ASIA-ISCoS Scientific Meeting, Boston, MA, June.
- Tobias CA, Han SS, Shumsky JS, et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 2005;22:138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;184:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Expression of the netrin receptor UNC-5 in lamprey brain: modulation by spinal cord transaction. Neurorehabil Neural Repair. 2000;14:49–58. doi: 10.1177/154596830001400106. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transaction. Biomaterials. 2006;27:519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transaction. J Neurotrauma. 2004;21:789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Krassioukov AV, Tator CH. Corticospinal regeneration into lumbar grey matter correlates with locomotor recovery after complete spinal cord transaction and repair with peripheral nerve grafts, fibroblast growth factor 1, fibrin glue, and spinal fusion. J Neuropathol Exp Neurol. 2005;64:230–244. doi: 10.1093/jnen/64.3.230. [DOI] [PubMed] [Google Scholar]
- Brosamie C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Anderson A, Horner PJ, Federoff HJ, Mendell LM. Combined delivery of neurotrophin-3 and NMDA receptors 2D subunit strengthens synaptic transmission in contused and staggered double hemi-sected spinal cord of neonatal rat. Exp Neurol. 2006;197:347–352. doi: 10.1016/j.expneurol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Salie R, Steeves JD. IGF-1 and BDNF promote chick bulbospinal neurite outgrowth in vitro. Int J Dev Neurosci. 2005;23:587–598. doi: 10.1016/j.ijdevneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Rochkind S, Shahar A, Amon M, Nevo Z. Transplantation of embryonal spinal cord nerve cells cultured on biodegradable microcarriers followed by low power laser irradiation for the treatment of traumatic paraplegia in rats. Neurol Res. 2002;24:355–360. doi: 10.1179/016164102101200131. [DOI] [PubMed] [Google Scholar]
- Guest J, Herrera LP, Qian T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord. 2006;44:135–142. doi: 10.1038/sj.sc.3101820. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Tang X, Denning JW, Archibald SJ, Davis SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–1242. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Ramer MS, Steeves JD. Setting the stage for functional repair of spinal cord injuries: a cast of thousands. Spinal Cord. 2005;43:134–161. doi: 10.1038/sj.sc.3101715. [DOI] [PubMed] [Google Scholar]