Abstract
Background/Objective:
Syringomyelia is characterized by a fluid-filled cavity within the spinal cord. While its pathogenesis is currently debated, the relationship of syringomyelia with other conditions, such as Chiari I malformation and cord/column trauma, is well accepted. Despite these common associations, a nidus for syrinx formation has not been identified in a subset of patients. We report 2 patients with idiopathic cervicothoracic syringomyelia who presented with progressive neurologic dysfunction. Diagnostic and treatment algorithms used in the care of these patients are presented.
Methods:
Retrospective review, including preoperative and postoperative studies, intraoperative findings, and the patients' surgical outcomes.
Results:
Patients underwent laminectomy, lysis of adhesions, untethering of spinal cord, fenestration of syrinx, and duraplasty after preoperative studies demonstrated evidence of focal cerebrospinal fluid flow block at the level of the syrinx. One patient's neurologic condition improved after surgery, whereas the other's remained unchanged without further deterioration; both showed radiographic decrease in the syrinx on immediate postoperative magnetic resonance imaging.
Conclusions:
These 2 cases illustrate patients who develop a cervicothoracic syrinx in the absence of any trauma, infection, previous manipulation of the neuraxis, or malformations known to be associated with a syringomyelia. Whereas there is no consensus on the optimal management of these patients, the patients reported here experienced arrest in deterioration or improvement of their neurologic examination, making the identification of this condition important as a potentially reversible cause of neurologic deficits. Long-term follow-up is required to determine the efficacy, durability, and lifestyle impact of the procedure.
Keywords: Spinal cord injuries, Syringomyelia, idiopathic, Syrinx, Arachnoiditis, Central subarachnoid stenosis
INTRODUCTION
Syringomyelia is a condition characterized by formation of a fluid-filled cavity within the spinal cord. Expansion of the cavity often results in a clinical course of progressive neurologic deficit, including pain, sensory loss, weakness, and autonomic dysfunction (1). The estimated annual incidence of this condition is 8.4 new cases/100,000 people (2). Whereas the pathogenesis of syrinx formation is still debated, its association with a number of conditions, including Chiari I malformation, spinal cord trauma, basilar impression, and occipital encephalocele, is well established (1,3–5). Despite these common associations, historical and clinical investigation has failed to identify a nidus for syrinx formation in a small minority of patients (6). We report 2 such patients with idiopathic syringomyelia who presented with progressive neurologic dysfunction; surgical intervention resulted in improvement or stabilization of clinical symptoms. The authors' previous experience with a population of posttraumatic syringomyelia demonstrated a common histologic feature of arachnoiditis and central subarachnoid stenosis; diagnostic and therapeutic algorithms used for the radiographic identification of cerebrospinal fluid (CSF) block and surgical reconstitution of physiologic flow patterns have been used with effect in this population (7). We postulate that the application of similar diagnostic and therapeutic algorithms will have an effect in cases of idiopathic syringomyelia.
CASE REPORTS
Patient 1
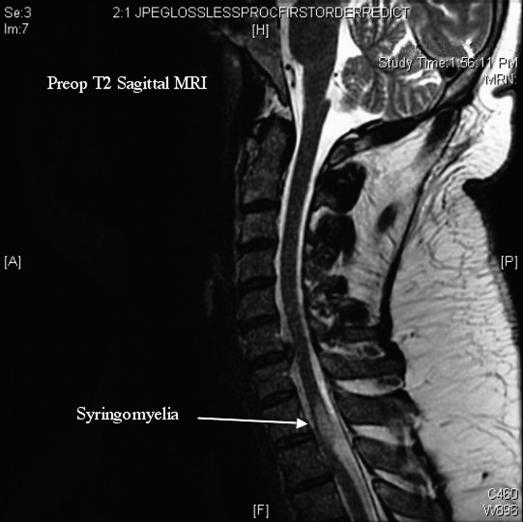
Presurgical Evaluation. Patient #1 was a 43-year-old former college football player without a history of spinal column or cord injury, infection, or other pathologic process who presented with severe neck pain and a recent history of right C7 radiculopathy. Upon questioning, he also admitted to a several year history of gait unsteadiness and urinary urgency. Physical examination revealed 5/5 strength in all muscle groups. Reflexes were 2+ at the biceps and brachioradialis, 1 at the triceps, 3 at the knees, and 2 at the ankles; no pathologic reflexes were present. The patient was frankly ataxic on tandem gait testing. Magnetic resonance imaging (MRI) revealed thoracic syringomyelia at T1 to T2 and minimally herniated disks at C5 to C6 and C6 to C7 (Figure 1). Cine MRI demonstrated focal restriction of CSF flow dorsally at the T1 to T2 level, while computed tomographic (CT) myelography revealed decreased contrast flow at T1 to T2 with ventral displacement of the spinal cord. Somatosensory evoked potentials (SSEP) studies revealed normal median evoked peripheral and central responses; the posterior tibial central responses were delayed, and the peripheral responses were unremarkable. SSEP findings were consistent with central disruption of the large-fiber afferent sensory pathways mediated through the posterior columns.
Figure 1. Patient 1. Preoperative MRI showing T2 syrinx.
Operative Course. Evidence of the thoracic syringomyelia with focal CSF flow block and cord compression, in the setting of progressive back pain and lower-extremity weakness, led to a recommendation of surgical treatment for the arrest of neurologic decline. T1 to T3 laminectomy and dural opening revealed impressive arachnoid scarring with the creation of a dorsal arachnoid cyst; there was no free flow of CSF. Arachnoid adhesions were lysed until free flow of CSF was noted both proximally and distally. The syrinx was fenestrated, the cord de-tethered, and redundant scar excised. Duraplasty was performed. Intraoperative neuromonitoring was unchanged.
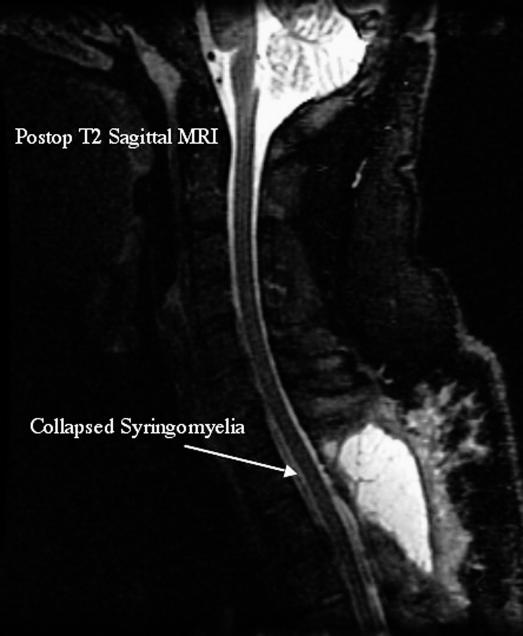
Postoperative Course. A decrease in syrinx size was apparent on immediate postoperative MRI. At 6 weeks, the patient had no complaints of lower-extremity pain, paresis, paresthesias, ataxia, or urinary urgency; C7 radiculopathy had resolved before surgery. At 3 months, the patient remained asymptomatic and had returned to work. Follow-up MRI at 1 year postoperatively demonstrated no recurrence of syrinx (Figure 2).
Figure 2. Patient 1. Five-month postoperative MRI showing resolution of syrinx.
Patient 2
Presurgical Evaluation. Patient 2 was a 45-year-old male choreographer without a history of column or cord trauma, infection, or other pathologic processes who presented with progressive left leg paresis and decreased sensation on the right side of his body from his abdomen to his thigh. Physical examination revealed trace weakness of the left leg and decreased sensation to pinprick in the right leg without a clear sensory level. Upper- and lower-extremity reflexes were 1+ and 3+, respectively. MRI showed extensive syringomyelia from C5 to T5, myelomalacia distal to T5, and a focal compartmentalization of the subarachnoid space from T4 through T6. Cine MRI revealed a lack of CSF flow dorsal to the cord from C5 to T1; no CSF flow was seen ventral to the cord from T1 through T5. CT myelography corroborated these findings. SSEP studies revealed normal median and tibial responses.
Surgical Procedure. T4 to T6 laminectomy and dural opening revealed a thickened and scarred arachnoid layer. A dorsal arachnoid space loculation, or cyst, compressed the cord. Adhesions were lysed and the dorsal arachnoid cyst was fenestrated. Upon completion of this, CSF flow was grossly noted from a proximal to a distal direction and vice versa. Duraplasty was performed. Intraoperative neuromonitoring was unchanged.
Postoperative Course. Immediate postoperative MRI showed interval decrease in the size of the syrinx; despite this, the neurologic examination was unchanged. The patient returned to work 2 months later. Five-month MRI showed refilling of the syrinx to baseline appearance. At 7 months, the syrinx was again stable by MRI. At that time, CT myelography demonstrated improved flow; cine MRI revealed dorsal CSF flow to the T3 level and ventral CSF flow to the T1 level. No flow was seen caudally from T4 through T7. The patient's clinical examination remained stable.
DISCUSSION
Classification
Syringomyelia is found most commonly in association with spinal or posterior fossa pathology. Two variations have been described: the first is a CSF-filled cystic cavitation within the central canal in communication with the fourth ventricle, and the second type is syrinx formation within the cord parenchyma that may or may not extend secondarily to the central canal (1,3,5,8). The former type, sometimes designated as hydromyelia, is far less frequent and is most often seen in children as the result of hydrocephalus and increased fourth ventricle pressure (3). These cavities are typically lined with ependymal tissue and contain fluid consistent with CSF (9).
More common is primary syrinx formation within the cord parenchyma, a condition often seen in association with foramen magnum or spinal pathologies that impede CSF flow, including Chiari I malformation, cervical stenosis, arachnoiditis, basilar impression, and occipital encephalocele (3). This type of syringomyelia is also seen in spinal cord/column injury and postinfectious arachnoiditis. Syrinx formation in this setting is usually localized to the central gray matter at the watershed zone between anterior and posterior cord vasculature and is lined with thick connective tissue, glia, or ependymal tissue (1,3,9).
The pathophysiology of syringomyelia is currently debated, and several hypotheses may account for the various subtypes. A widely held theory is that local CSF flow is disturbed within the subarachnoid compartment, creating a pressure gradient that drives fluid across the perivascular space, thus increasing fluid volume within the extracellular compartment (10,11). In a different manner with a similar outcome, arachnoiditis and other etiologies may scar over pial fenestrations, thereby blocking extracellular outflow to the subarachnoid space. The excess fluid load organizes into a longitudinal syrinx extending over multiple cord segments (5,8).
Numerous aspects of syringomyelia formation and maintenance are not adequately explained by previous theories. One recent study proposes that certain maneuvers, such as an upright posture or coughing, are thought to produce a transient pressure gradient across the CSF flow block (9). Subsequent vascular dilation and compression impart repeated hydrodynamic stress to the cord, culminating in tissue breakdown and expression of a protein-poor crystalloid material into the cord parenchyma. Slow buildup of this fluid both creates and maintains the syrinx.
Our patients did not have the history of trauma or neuraxis pathology typically seen in those with syringomyelia, and classic foramen magnum/hindbrain pathology was absent on radiographic evaluation. It is of interest that each patient had been involved in activities in which subtle microtrauma to the cord may have been sustained. The few studies that have examined such idiopathic syrinx development have focused on the presence of subclinical foramen magnum obstruction to CSF flow. Bogdanov et al compared the clinical features and posterior fossa measurements among 3 populations, including 17 patients with idiopathic syringomyelia, 17 patients with Chiari I malformations, and a control population of 32 subjects (12). Measurement of the posterior fossa and foramen magnum demonstrated similar morphometric abnormalities in the Chiari and idiopathic groups, including tight confinement of the hindbrain and narrow CSF spaces. Likewise, both groups had similar presentations of central cervical myelopathy. The authors postulated that intradural adhesions and osseus confinement within an anatomically “tight” posterior fossa (the “Chiari zero malformation”) produced accentuated pulsatile CSF subarachnoid pressure waves that lead to syrinx development. A similar study by Kyoshima et al showed that idiopathic syringomyelia was associated with a narrowed foramen magnum that could prevent adequate fourth ventricle venting of physiologic CSF pulsations (1). Both of these investigations concluded that idiopathic syringomyelia is formed in the presence of small anatomic aberrations within the spinal canal or at the craniocervical junction.
Diagnosis
Both patients presented with progressive neurologic symptoms of pain, sensory loss, and lower-extremity paresis. Clinical symptoms will vary, depending on a syrinx's longitudinal location along the neuraxis as well as its position within the cord. Syrinx expansion can produce both an exacerbation of existing symptoms as well as new clinical deficits (3). Classically, the first manifestation is pain, followed by paresis, paresthesias, and sphincter dysfunction (13). One study found that sensory disturbance was the most common presentation in a small population of idiopathic syringomyelia patients; less common symptoms were pain, weakness, and sphincter disturbance (6). Pain manifestations are varied and include pain localized to the syrinx site, central-type pain, or a radicular pain. Posture change or Valsalva-like maneuver can exacerbate symptoms, a phenomenon that is consistent with the hydrodynamic theories of syringomyelia genesis. Sensory disturbances can appear in a “cape-like distribution,” in a pattern of an ascending sensory level, or as a dissociated sensory loss. Upper motor neuron signs in the legs may contrast with a lower motor neuron portrait in the arms. Rostral extension of the lesion may produce bulbar symptoms, resulting in possible aspiration, cyanosis, stridor, or apnea (9,13).
Given its dynamic nature, syringomyelia should be considered in the differential of any patient with progressive neurologic deficit; in patients with prior cord or column trauma, syringomyelia is considered the cause of new-onset neurologic dysfunction until proven otherwise (14). MRI is an excellent modality for identification of the syrinx and accompanying pathology. T1 and T2 relaxation characteristics of the syrinx fluid suggest a nonenhancing CSF-like picture (7,15); T2 hyperintensity within the cord parenchyma adjacent to the cavity may correlate with myelin degeneration and edema formation, with accompanying gliosis (15). Imaging has confirmed that the majority of cases are caused by lesions obstructing CSF flow within the subarachnoid space (12).
Analysis of CSF flow characteristics by cine MRI or CT myelography is an important step in the quantification and localization of the subarachnoid CSF flow block. CT myelography can precisely guide surgical interventions by revealing arachnoid webs, cysts, and loculations that impede physiologic CSF flow, thus leading to syrinx formation. The aforementioned lesions are often missed on conventional MRI (6,8,16). Cine MRI has demonstrated efficacy in the evaluation of CSF flow characteristics in a variety of spinal column pathologies, including both Chiari malformation and syringomyelia. In cine MRI, sagittal images are obtained with phase contrast sequences; as such, cine MRI can identify and localize obstructions to CSF flow (16). Through the evaluation of CSF velocity and waveforms in both the preoperative and postoperative settings, restoration of physiologic flow patterns has proven to be an important prognostic factor for symptom resolution (17).
Radiologic improvement or resolution of the syrinx after operative intervention does not necessarily correlate with clinical improvement. Our second patient demonstrated initial collapse of the thoracic syrinx on early postoperative imaging, yet there was no change in symptoms. Mechanisms for this phenomenon are unknown; theories have focused on an irreversible neuronal/axonal insult or progressive gliosis of the collapsed walls of the syrinx (14,16). In contrast, some patients may experience improvement of clinical symptoms in the absence of complete syrinx resolution. This can occur when surgical intervention is directed toward optimization of CSF flow rather than drainage of the cavity. Postoperative flow studies should ideally reveal correction of subarachnoid block and increased velocity of CSF flow (16). Within this same vein, failure to identify a flow abnormality on preoperative functional imaging may serve as a poor prognostic factor for the surgical resolution of clinical symptoms (18).
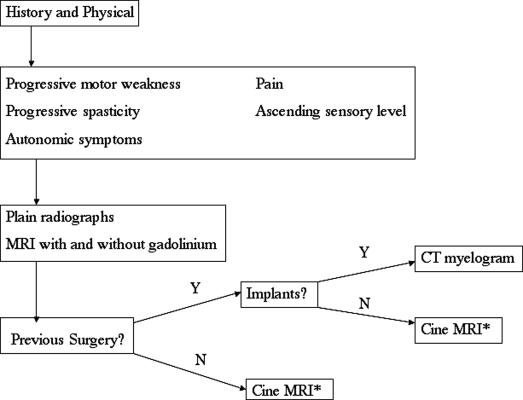
Our own diagnostic algorithm (Figure 3) for suspected idiopathic syringomyelia, derived from experiences with posttraumatic syringomyelia (7), begins with any patient with a progressive motor weakness or spasticity, autonomic dysfunction, pain, or an ascending sensory level. Baseline spinal axis imaging is obtained, including plain radiography and MRI with and without gadolinium. When evidence of syringomyelia is noted, CSF flow is assessed initially via cine MRI for patients without a history of previous surgery or spinal implants. All patients are evaluated by computerized tomographic (CT) myelography.
Figure 3. Diagnostic algorithm for syringomyelia. *May require CT myelogram for exact localization of pathology.
Treatment
Surgical treatment of syringomyelia has evolved in the past century; however, optimal management is still not fully defined. It is increasingly clear that, while correction must be tailored toward individual syrinx etiology, the unifying intention should be the amelioration of filling defects rather than simple shunting and drainage of ever-evolving cavitations (3). Various shunting strategies, including syringosubarachnoid, syringoperitoneal, and syringopleural drainage, all share the same basic goal of syrinx collapse and reduction of cord parenchymal compression. Whereas authors have demonstrated short-term clinical and radiographic improvement, long-term retrospective analysis has shown both a high rate of failure to effect adequate syrinx resolution, as well as shunt-related morbidities such as infection and catheter tip migration (19,20). Much of the failure of shunting is caused by the reactive gliosis induced by both surgical placement and by shear stress on the cord tissue from the anchored catheter (6,19,20). Obstruction of the catheter lumen by glial ingrowth or syrinx collapse around the shunt tip results in a reexpansion of the original syrinx or growth of an adjacent cavitation as the syrinx filling mechanism persists. Scarring produced by the mechanical stress of an indwelling shunt can tether the cord and cause further abnormal CSF flow.
Better long-term results are obtained when any filling abnormalities are surgically disabled and CSF pathways are opened so as to bypass flow into the syrinx (6,9,20). In those with Chiari malformations, small posterior fossa, or tight cisterna magna (1,12), the most favorable management involves decompression of the foramen magnum accompanied by high cervical laminectomy and intradural exploration with lysis of arachnoid adhesions. This intervention will achieve the dual purpose of correcting the fluid-pressure differential across the foramen magnum and relieving the subarachnoid stenosis (12,16,19).
When presurgical imaging does not identify foramen magnum pathology, targeting the flow block revealed by cine MRI or CT myelogram and reconstructing the subarachnoid space can achieve a high level of success in correcting the instigating pathology and syrinx maintenance mechanisms. Reconstruction, as was performed for our 2 patients and outlined in our management algorithm, involves varied combinations of multilevel laminectomy, dural opening, lysis of adhesions, untethering of the cord, fenestration of the syrinx, and augmentation of the subarachnoid space by duraplasty or the creation of a surgical meningocele (9,14,19). One study reported a success rate above 80% in the treatment of syringomyelia by reconstruction after 4 years of follow-up (14). While much of the evidence supporting subarachnoid reconstruction is derived from work on patients with posttraumatic syringomyelia, it seems intuitive that any evidence of flow block documented on preoperative studies would benefit from similar intervention. Other reports of CSF block reconstruction in patients with idiopathic syrinx showed improvement in radiographic and clinical evaluations (6,8).
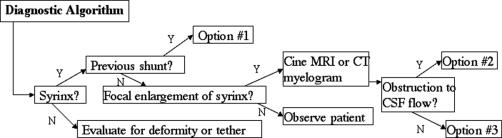
The therapeutic algorithm we propose for idiopathic syringomyelia is guided by previous treatment and radiographic findings (Figure 4) as well as by experience with a large cohort of patients with posttraumatic syringomyelia (7). Symptomatic syringomyelia with a history of shunt placement is managed with lysis of arachnoid adhesions, syrinx fenestration, removal of the shunt, and duraplasty. For patients without a history of shunting, radiographic demonstration of CSF flow obstruction on CT myelography is treated with lysis of arachnoid adhesions, syrinx fenestration, and duraplasty. For those with symptomatic syringomyelia without any evidence of CSF flow obstruction, surgical exploration is offered as an option.
Figure 4. Treatment algorithm for syringomyelia. *See diagnostic algorithm (Figure 3). Surgical treatments include: Option #1: lysis of adhesions, syrinx fenestration, removal of shunt, duraplasty; Option #2: lysis of adhesions, syrinx fenestration, duraplasty; and Option #3: possible exploration at site of initial injury.
CONCLUSION
Idiopathic syringomyelia presents a particular challenge to the neurosurgeon if one considers the amelioration of syrinx etiology as the goal of surgical intervention, rather than simply palliation through shunting of cord fluid collections. Cervical tether release or foramen magnum decompression in the setting of Chiari malformation are pragmatic interventions targeting the syrinx mechanism, but idiopathic syrinx eludes easily identifiable correction. Nevertheless, the reestablishment of CSF flow in the proximity of syrinx has been demonstrated in this and other reports as an optimal intervention strategy that allows for improvement or stabilization of symptoms that might otherwise progress to severe pain or disabling neurologic impairment. Structured and complete neur-axis imaging and evaluation of CSF flow characteristics allow for a targeted surgical strategy. Whereas both the diagnostic and treatment algorithms presented here have been used with success in the posttraumatic population, long-term analysis of their application to idiopathic syringomyelia by both radiographic and clinical outcomes will yield data assessing the best timing and mode of surgical intervention.
REFERENCES
- Kyoshima K, Kuroyanagi T, Oya F, Kamijo Y, El-Noamany H, Kobayashi S. Syringomyelia without hindbrain herniation: tight cisterna magna. Report of four cases and a review of the literature. J Neurosurg. 2002;96:239–249. doi: 10.3171/spi.2002.96.2.0239. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo N, Cacciola F. Adult syringomyelia. Classification, pathogenesis and therapeutic approaches. J Neurosurg Sci. 2005;49(3):65–72. [PubMed] [Google Scholar]
- Milhorat TH, Capocelli AL, Jr, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringo-myelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- Pare LS, Batzdorf U. Syringomyelia persistence after Chiari decompression as a result of pseudomeningocele formation: implications for syrinx pathogenesis: report of three cases. Neurosurgery. 1998;43:945–948. doi: 10.1097/00006123-199810000-00125. [DOI] [PubMed] [Google Scholar]
- Klekamp J, Iaconetta G, Batzdorf U, Samii M. Syringomyelia associated with foramen magnum arachnoiditis. J Neuro-surg. 2002;97:317–322. doi: 10.3171/spi.2002.97.3.0317. [DOI] [PubMed] [Google Scholar]
- Mallucci CL, Stacey RJ, Miles JB, Williams B. Idiopathic syringomyelia and the importance of occult arachnoid webs, pouches and cysts. Br J Neurosurg. 1997;11:306–309. doi: 10.1080/02688699746087. [DOI] [PubMed] [Google Scholar]
- Stadler JA, Ganju A. Surgical treatment of posttraumatic syringomyelia [abstract #86] J Spinal Cord Med. 2006;29:282. [Google Scholar]
- Bruneau M, Duprez T, Rommel D, Raftopoulos C. Surgical treatment of a syringomyelia associated with an idiopathic arachnoid malformation disclosed by preoperative MRI. Surg Neurol. 2004;62(6):552–525. doi: 10.1016/j.surneu.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Levine DN. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: a critical review of existing theories and proposal of a new hypothesis. J Neurol Sci. 2004;220(1–2):3–21. doi: 10.1016/j.jns.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Brodbelt AR, Stoodley MA, Watling AM, Tu J, Jones NR. Fluid flow in an animal model of post-traumatic syringo-myelia. Eur Spine J. 2003;12:300–306. doi: 10.1007/s00586-002-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91(4):553–562. doi: 10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- Bogdanov EI, Heiss JD, Mendelevich EG, Mikhaylov IM, Haass A. Clinical and neuroimaging features of ''idiopathic'' syringomyelia. Neurology. 2004;62:791–794. doi: 10.1212/01.wnl.0000113746.47997.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier AB, Foo D, Shillito J, Dyro FM. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108(Pt 2):439–461. doi: 10.1093/brain/108.2.439. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Williams B. Management and outcome of posttraumatic syringomyelia. J Neurosurg. 1996;85:197–205. doi: 10.3171/jns.1996.85.2.0197. [DOI] [PubMed] [Google Scholar]
- Jinkins JR, Reddy S, Leite CC, Bazan C, III, Xiong L. MR of parenchymal spinal cord signal change as a sign of active advancement in clinically progressive posttraumatic syrin-gomyelia. Am J Neuroradiol. 1998;19:177–182. [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chung CK, Kim HJ. Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation. Spinal Cord. 2002;40:501–506. doi: 10.1038/sj.sc.3101322. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Watabe N, Takahashi T, Shimizu H, Yoshimoto T. Quantitative assessment of surgical decompression of the cervical spine with cine phase contrast magnetic resonance imaging. Neurosurgery. 2002;50:791–796. doi: 10.1097/00006123-200204000-00020. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Nimjee SM, Fuchs HE, George TM. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery. 2006;59(1):140–146. doi: 10.1227/01.NEU.0000219841.73999.B3. [DOI] [PubMed] [Google Scholar]
- Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382–388. doi: 10.3171/jns.1998.89.3.0382. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1–10. doi: 10.3171/jns.1995.82.1.0001. [DOI] [PubMed] [Google Scholar]