Abstract
Case report:
A 25-year-old man with Behçet's disease was admitted because of weakness of the lower limbs and difficulty in urination. He had received a rabies vaccination 2 months previous because he had been bitten by a dog.
Findings:
Clinical and laboratory findings supported acute transverse myelitis. A hyperintense lesion and expansion at the level of conus medullaris was detected on spinal magnetic resonance imaging.
Conclusion:
Although neurologic involvement is one of the main causes of mortality and morbidity in Behçet's disease, the factors that aggravate the involvement of the nervous system are still unclear. Vaccination may have been the factor that had activated autoimmune mechanisms in this case. To our knowledge, involvement of the conus medullaris in Behçet's disease after rabies vaccination has not been reported.
Keywords: Rabies, Vaccination, Acute transverse myelitis, Behçet's disease, Conus medullaris, Neuro-Behçet syndrome
INTRODUCTION
Transverse myelitis is a clinical syndrome that involves the spinal cord, resulting in varying degrees of weakness, sensory alterations, and autonomic dysfunction (1). Acute transverse myelitis (ATM) can be due to post-infectious immune-mediated inflammation of the spinal cord (2). Active or passive immunization with vaccines or sera can cause lesions with immunomediated pathogenesis and may involve both the central and the peripheral nervous system (3). Vaccination as an etiology of ATM is frequently reported, but the involvement of the conus medullaris after vaccination has been reported in only one case (4).
Behçet's disease is a well-known multisystemic vasculitis, but the etiology is still not well understood. Some factors (eg, autoimmunity, infections, heredity, environmental factors) have been frequently described in the etiopathogenesis (5), but only one case of Behçet's disease triggered by vaccination has been reported previously (6). The neurological involvement in Behçet's disease is well known, but myelitis is rare, especially when it is the only neurological manifestation (7). The factors causing or activating the neurological involvement in Behçet's disease are still not clear. We report a patient with Behçet's disease presenting with a conus medullaris lesion in whom the involvement may be associated with rabies vaccination.
CASE REPORT
A 25-year-old man with Behçet's disease was admitted because of weakness of his lower limbs and urinary retention. He had a severe backache for 12 hours before the onset of lower-limb weakness. His weakness progressively worsened, and he began experiencing difficulty with urination and defecation. He had received a rabies vaccination 2 months earlier after being bit by a dog. Rabipur (active substance: inactivated rabies virus) was administered on the day of the bite and on the 3rd and 7th days thereafter. Past medical history revealed that he had recurrent aphthous stomatitis and genital ulcerations beginning at age 13 years. He had been diagnosed with Behçet's disease when he was 20 years old. The pathergy test was positive. He also had an attack of deep-venous thrombosis 2 years previous.
On physical examination, a few papulopustular lesions inside the thighs and genital ulceration were found. His muscle strength was 2/5 at his proximal left lower limb, 1/5 at his distal left lower limb, 3/−5 at his proximal right lower limb, and 1/5 at his distal right lower limb in Medical Research Council grade. There was hypoesthesia below the level of L1, and his vibratory and position senses were absent in his lower extremities. Deep tendon reflexes were absent in the lower extremities, and plantar responses were extensor bilaterally. The remainder of the neurological examination was normal.
Diagnostic Workup
Complete blood count, biochemistry, and sedimentation rate were normal. Serum hepatitis markers, anti–human immunodeficiency virus (HIV) antibody (Ab), Brucella agglutination test, Venereal Disease Research Laboratory test, varicella zoster virus (VZV) Ab, and Toxoplasma Ab were negative. Markers of vasculitis, including the autoantibodies SS-A, SS-B, anti-nuclear antibody (ANA), and anti-DNA, were negative.
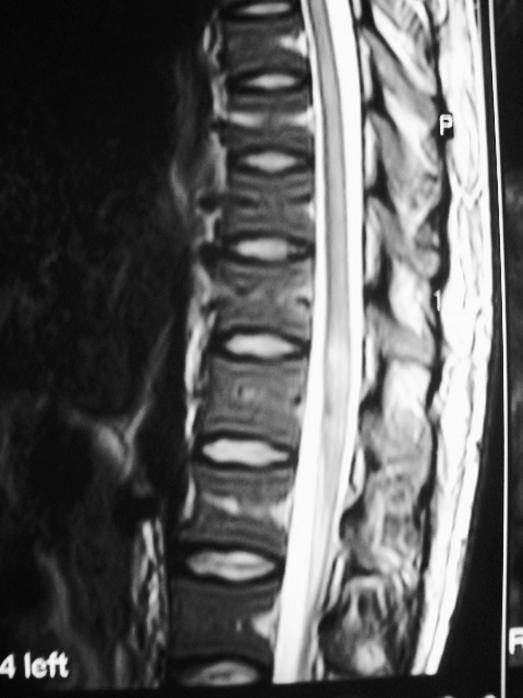
The patient's cerebrospinal fluid (CSF) was macroscopically clear; microscopic examination showed 110 leucocytes/mm3, and no microorganisms were found. The CSF protein level was elevated (114 mg/dL), glucose level was 44 mg/dL, and simultaneous serum glucose level was 70 mg/dL. Oligoclonal banding was not found in the CSF, and the CSF IgG index was 0.59 (N < 0.66). Total Toxoplasma Ab, cytomegalovirus (CMV) IgM and IgG Ab, herpes simplex virus (HSV) types I and II IgM, and HSV types I and II IgG Ab were negative in CSF. Magnetic resonance imaging (MRI) of the cranium was normal. A hyperintense lesion and expansion at the level of the conus medullaris was detected in his spinal MRI (Figure 1). It was Gd(t). Computed tomographic angiography of the vascular structures, cardiology consultation, echocardiography, and hematological investigations were performed in detail, and all were in normal limits.
Figure 1. Initial spinal magnetic resonance image showing a hyperintense lesion and expansion at the level of the conus medullaris (T2W).
After hospitalization, pulse steroid therapy of 1g/d for 5 days was started. After pulse methylprednisolone therapy was switched to oral form and dosages were decreased slowly, azathioprine 150 mg/d was added to his treatment and continued for long-term therapy.
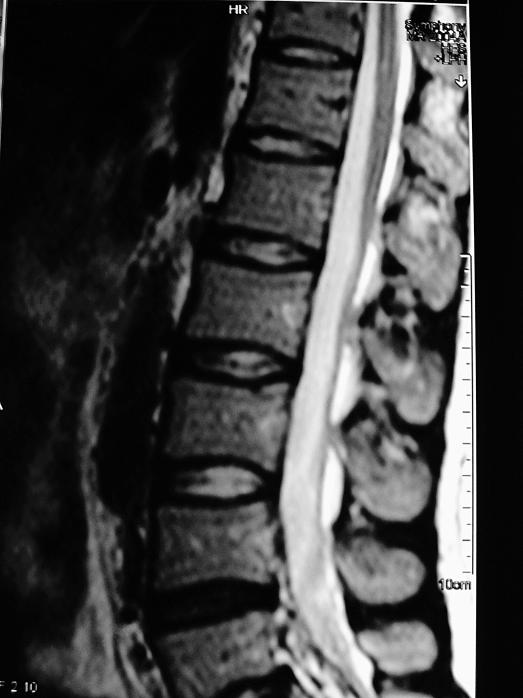
The patient underwent regular physiotherapy, and after 1 month his muscle strength was 3+/5 in his left lower limb and 4/5 in his right lower limb. Atrophy and fasciculations were seen, especially in his left leg muscles innervated by L5-S1. There was hypoesthesia below level L3. His vibratory and position senses and deep tendon reflexes were absent at his lower extremities. Plantar responses were extensor bilaterally. The patient was transferred to a physiotherapy center. Urinary retention and detrusor atony were detected in urodynamic studies. He needed intermittent catheterization for 4 months. One year later, his complaints and pathologic findings had completely resolved. Control spinal MRI was normal (Figure 2).
Figure 2. Follow-up spinal magnetic resonance image showing resolution, taken 1 year later when symptoms had resolved.
DISCUSSION
The neurological complications of Behçet's disease are observed in 5 to 35% of all cases, depending on the series (8). Tohme et al (9) studied central nervous system and peripheral nervous system involvement in Behçet's disease, and they found that meningoencephalitis and/or transverse myelitis were the most frequent features (69%) followed by tumor-like manifestations (13%). However, transverse myelitis is rare, especially as a sole clinical manifestation (8). This patient's diagnosis of Behçet's disease was confirmed at our clinic, and he had only ATM as the presenting neurological manifestation.
Acute transverse myelitis is an inflammatory disorder. The pathogenesis is unclear, but the probable mechanism involves an autoimmune phenomenon. Possible causes include parainfectious and postvaccination events; ATM also can be seen in the course of multiple sclerosis (10). Das et al (11) reported the etiologies of transverse myelitis or myelopathy and found the following: 29.26% postinfectious, 19.51% demyelination, 3.65% vascular and vasculitis, 1.21% toxic, and 2.42% physical. They also reported a vaccination history in 1.21% of their patients, as in this case, in which a rabies vaccination was given 2 months prior to the onset of neurological symptoms.
Acute transverse myelitis may be an isolated entity or may occur in the context of a multifocal or even a multisystemic disease (12). In this case, 2 different factors (Behçet's disease and vaccination) may explain the etiology of ATM. Carod-Artal et al (13) and Olson et al (14) reported cases with myelopathy at the level of the conus medullaris due to Schistosomia mansoni. Longitudinal involvement of the spinal cord including the conus medullaris level due to systemic lupus erythematosus-related transverse myelitis has been reported (15,16). However, it has not been reported with Behçet's disease or after rabies vaccination. Only one case with isolated myelitis of the conus medullaris due to vaccination against tetanus-poliomyelitis was reported by Abdennebi et al (4).
Although neurologic involvement is one of the main causes of mortality and morbidity in Behçet's disease, the factors that aggravate the involvement of the nervous system are still unclear. In this case, vaccination may be the factor that activated autoimmune mechanisms and may have caused the neurological involvement. There have been no controlled drug trials for treatment of Neuro-Behçet syndrome, but steroids and immunosuppressive can be beneficial.
CONCLUSION
Involvement of the conus medullaris in Behçet's disease after rabies vaccination has not been reported previously in the literature. The possibility of an activating factor (eg, vaccination) other than infection has never been mentioned in Neuro-Behçet syndrome. Understanding the activating factors may be helpful for the prevention of neurological involvement and is important for planning follow up and understanding the prognosis. Further reports and investigations are needed.
REFERENCES
- Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA. Transverse myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483–1499. doi: 10.2741/1351. [DOI] [PubMed] [Google Scholar]
- Chan KH, Tsang KL, Fong GC, Cheung RT, Ho SL. Idiopathic severe recurrent transverse myelitis: a restricted variant of neuromyelitis optica. Clin Neurol Neurosurg. 2005;107:132–135. doi: 10.1016/j.clineuro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tezzon F, Tomelleri P, Ferrari G, Sergei A. Acute radiculo-myelitis after antitetanus vaccination. Ital J Neurol Sci. 1994;15:191–193. doi: 10.1007/BF02339322. [DOI] [PubMed] [Google Scholar]
- Abdennebi A, Dumas JL, Salama J, Benromdhane H, Belin C, Goldlust D. Postvaccination myelitis: aspect and course followed by MRI. J Radiol. 1996;77:363–366. [PubMed] [Google Scholar]
- Evereklioglu C. Current concepts in the etiology and treatment of Behçet disease. Surv Ophthalmol. 2005;50:297–350. doi: 10.1016/j.survophthal.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Molloy ES, Powell FC, Doran MF, et al. An unusual case of Behçet's syndrome: triggered by typhoid vaccination? Clin Exp Rheumatol. 2004;22(suppl):71–74. [PubMed] [Google Scholar]
- Harmouche H, Mouti O, el-Alaoui Faris M, Aidi S, Benabdeljalil M, Chkili T. Acute myelitis and Behçet's disease: three case reports [in French] Rev Med Interne. 2000;21:1047–1051. doi: 10.1016/s0248-8663(00)00265-4. [DOI] [PubMed] [Google Scholar]
- Wechsler B, Sbai A, Du-Boutin LT, Duhaut P, Dormont D, Piette JC. Neurological manifestations of Behçet's disease. Rev Neurol. 2002;158:926–933. [PubMed] [Google Scholar]
- Tohme A, Haddad F, Ghayad E. Neurologic manifestations in Behçet's disease: 16 cases in a cohort of 110 patients. Ann Intern Med. 1997;148:118–124. [PubMed] [Google Scholar]
- Iniguez C, Mauri JA, Larrode P, Lopez del Val J, Jerico I, Morales F. Acute transverse myelitis secondary to hepatitis B vaccination. Rev Neurol. 2000;31:430–432. [PubMed] [Google Scholar]
- Das K, Saha SP, Das SK, et al. Profile of non-compressive myelopathy in eastern India: a 2-year study. Acta Neurol Scand. 1999;99:100–105. doi: 10.1111/j.1600-0404.1999.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Kerr DA, Ayetey H. Immunopathogenesis of acute transverse myelitis. Curr Opin Neurol. 2002;15:339–347. doi: 10.1097/00019052-200206000-00019. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ, Vargas AP. Myelopathy due to Schistosoma mansoni: a description of two cases and review of the literature. Rev Neurol. 2004;39:137–141. [PubMed] [Google Scholar]
- Olson S, Rossato R, Guazzo E. Spinal schistosomiasis. J Clin Neurosci. 2002;9:317–320. doi: 10.1054/jocn.2001.0981. [DOI] [PubMed] [Google Scholar]
- Kimura KY, Seino Y, Hirayama Y, et al. Systemic lupus erythematosus related transverse myelitis presenting longitudinal involvement of the spinal cord. Intern Med. 2002;41:156–160. doi: 10.2169/internalmedicine.41.156. [DOI] [PubMed] [Google Scholar]
- Deodhar AA, Hochenedel T, Bennett RM. Longitudinal involvement of the spinal cord in a patient with lupus-related transverse myelitis. J Rheumatol. 1999;26:446–449. [PubMed] [Google Scholar]