Abstract
Objective:
To compare body composition in patients aged 11 to 21 years with spinal dysfunction due to spinal cord injury (SCI) and spina bifida (SB) vs able-bodied control (CTRL) and able-bodied overweight (OW) groups and to examine the relationships between resting energy expenditure (REE) and total lean mass (TLM) in the SCI, SB, CTRL, and OW groups.
Methods:
Two hundred fifteen subjects, including 85 CTRL, 31 OW, 33 SCI, and 66 SB, were evaluated. Body composition was estimated by dual energy x-ray absorptiometry (DXA). Measurements included height, weight, total lean mass (TLM), fat tissue mass (FTM), body mass index (BMI), BMI percentile (BMI%tile), and % fat. Resting energy measurements were obtained in fasting subjects with an open-circuit indirect calorimeter.
Results:
There were gender differences in height, weight, BMI, TLM, fat mass, % fat, and REE. The REE in the SCI and SB groups was significantly different from that in the CTRL and OW groups, but no significant difference was found between the SCI and SB groups. The SB group had significantly higher REE/TLM ratios than did the other groups. The % fat was significantly higher in the SB and OW groups as compared to the CTRL and SCI groups. TLM was significantly higher in CTRL and OW groups as compared to SCI and SB groups, with the lowest TLM found in the SB group.
Conclusion:
Patients aged 11 to 21 years with SB or SCI have significant lean tissue mass deficits by DXA as compared to able-bodied CTRL and OW groups, with the greatest deficits in total lean mass measured in SB. The absolute REE values were significantly reduced in both SCI and SB groups in association with their lean tissue deficits. Interestingly, REE/TLM ratios were remarkably constant in the CTRL, OW, and SCI groups but significantly elevated in the SB group. One would expect an even greater degree of adiposity in the SB group if their REE/TLM ratios were not elevated relative to those without congenital paralysis.
Keywords: Spina bifida; Body composition, weight control; Child; Adolescence; Obesity
INTRODUCTION
Obesity in children and adolescents is one of the most frustrating and difficult medical conditions to treat. Prevention represents the best treatment approach in both able-bodied and disabled populations. The prevalence of pediatric obesity in the United States is increasing at an accelerated rate. Current data from the National Health and Nutrition Examination Survey (NHANES) show the prevalence of overweight at 16% among both children 6 to 11 years old and adolescents 12 to 19 years old in the United States, which represent increases of nearly 50% compared to NHANES III data from 1984–96 and a threefold increase from the 1960s. The high prevalence of overweight is not limited to able-bodied children and adolescents; it also occurs in those with disabilities from spinal cord dysfunction (SCD).
Children and adolescents with SCD due to spina bifida (SB) have been documented by many investigators to have a high prevalence of obesity (1–8). The high prevalence of obesity in SB is not due to the primary disorder, a neural tube defect caused by the failure of the fetus's spine to close properly during the first month of pregnancy, but to secondary conditions that result from the disability. While prevalence estimates for obesity in children with SCD due to spinal cord injury (SCI) have not been published to date, obesity is common among adults with SCI (9,10). Patients with upper lumbar or thoracic SB and SCI experience locomotor impairment and restriction in the performance of their normal everyday activities, which lead to an increasingly sedentary lifestyle (11,12). This, in turn, can be associated with a decrease in physical fitness and an increase in body fat (13). Deficits in lean muscle tissue in adolescents with motor impairments such as SB and SCI are associated with a high prevalence of obesity and suboptimal physical fitness (13). Obesity can exacerbate problems with ambulation and transferring, pressure sores, psychological morbidity, and complications during surgery (14). Similar to the able-bodied population, obesity in adults with spinal dysfunction is associated with metabolic syndrome, which is a constellation of health risk factors, including glucose intolerance, hyper-lipidemia, and hypertension. These risk factors, in turn, are associated with increased prevalence of type II diabetes mellitus and coronary heart disease (15–17).
Several studies have used body mass index (BMI) to classify obesity. BMI, defined as the weight (kg) divided by the height (meters) squared, is frequently used in a clinical setting as a measure of body composition in children because it is simple, cost effective, and requires limited training (18,19). The American Academy of Pediatrics recommends that BMI for age be plotted and a percentile determined for all children on an annual basis as a screen for obesity (20). However, due to profound reduction in lean tissue mass, and significant increase in body fat in children with SCD as well as shorter stature in SB, it is questionable if BMI can be used to accurately predict adiposity in SCD.
A 2003 study by Littlewood and colleagues showed that anthropometric measurements in children with SB are significantly different from those of able-bodied children due to their short stature (21). Many investigators have found that body composition markedly changes during the first 6 months after SCI: total body lean tissue mass decreased as much as 9.5% by 6 months postinjury, whereas leg lean tissue mass decreased 15.1% by 1 year postinjury (22).
Accurate assessment of body composition, particularly measures of fat tissue, is a key component in the clinical treatment of childhood obesity. Various techniques have been used to assess body composition, including measurements of total body water, total body potassium, skinfold thicknesses, hydrostatic weighting, bioelectrical impedance, and dual energy x-ray absorptiometry (DXA). Each methodology has advantages and disadvantages based on cost, accessibility, compliance, equipment requirements, accuracy, and reliability. Because of its ease of use and accuracy, DXA has been shown to be one of the most feasible, valid, and reliable measures of body composition in people with disabilities (23–25). Both lean and fat tissue mass can be accurately and reliably estimated in children and adults with this method (26–28).
Several studies have focused on evaluating resting energy expenditure (REE) and its correlation with body composition in children and adolescents with SCD (29–31). Bandini and colleagues (29) suggested that children and adolescents with SB have reduced energy expenditure due to reduced lean body mass. A number of studies indicate that persons with SCI have low absolute REE (31–36). However, the relationship between REE and total lean mass (TLM) needs further investigation to improve the understanding of the relationship between energy expenditure and body composition in patients with SCD due to SB and SCI.
The major aims of this study are (a) to compare REE and body composition in patients age 11 to 21 years with SCD due to SB and SCI to those of able-bodied CTRL and able-bodied overweight (OW) groups; and (b) to examine the relationships between REE and TLM in the same groups.
METHODS
Subjects
A convenience sample of 215 young people age 11 to 21 years was included in the study. Thirty-three subjects with SCI and 66 with SB were recruited for this study from specialty clinics at the Shriners Hospital for Northern California and the University of California at Davis Medical Center. Eighty-five healthy, able-bodied normal-weight controls (CTRL) were recruited through fliers in the community and from friends of the patients. Thirty-one able-bodied overweight (OW) subjects were recruited from pediatric obesity clinics at the University of California at Davis Medical Center. All subjects and a parent/guardian (for subjects less than 18 years of age) provided written and oral consent and assent in accordance with the Institutional Review Board of the University of California at Davis. Subjects were instructed to fast and to abstain from intensive exercise for 12 hours prior to the day of testing. Measurements were obtained between 7:30 am and 9:30 am.
Anthropometric Measurement
Height was measured to the nearest 0.5 cm with a wall-mounted stadiometer, and body weight was measured to the nearest 0.5 kg with a balance scale (Detecto, Webb City, MO). All subjects reported feeling in good health on the days of testing.
BMI Calculation
Body mass index (BMI; kg/m2) was calculated by dividing weight in kilograms by the square of height in meters. Overweight status was defined using sex- and age-specific growth charts (BMI) developed by the Centers for Disease Control and Prevention, with normal weight defined as a BMI between the 5th percentile and <85th percentile, at risk of overweight defined as a BMI >85th percentile and <95th percentile, and overweight as >95th percentile. There is no specific distinction between overweight and obese categorizations for children and adolescents.
Body Composition
Body composition was estimated by DXA with the Hologic QDR4500A total body scanner (Hologic, Bedford, MA). To ensure quality control, the DXA unit was calibrated daily using a standard calibration block of thermoplastic acrylic resin, which contained 3 bone equivalent chambers filled with hydroxyapatite. Bone mineral density and content were required to be within 2% of the known values before data collection. All scans were performed and analyzed by a certified technician. Body composition measurements of bone mineral content (BMC), total lean tissue mass (TLM), and fat tissue mass (FTM) were determined using software algorithms based upon derived regression equations (37). Percent body fat by DXA for the total body was calculated using the formula 100 × FTM/(FTM + TLM + BMC).
Resting Energy Expenditure
Gas exchange measurements were obtained with an open-circuit indirect calorimeter (MedGraphics Corp, St Paul, MN) with a facemask while the subject lay in a darkened room at a comfortable temperature (22°C to 24°C). Subjects were instructed to lie as still and as relaxed as possible. Researchers made every effort to ensure that the subjects were comfortable and relaxed. Gas exchange data were recorded for 30 minutes, but only the data from the lowest 10 minutes were analyzed. The quality of the test was evaluated by determining whether the RQ was consistent with the patient's nutritional intake, whether RQ rested within the normal physiological range (0.67–1.3), whether measured VO2 was within ±10% of the mean value measured and VCO2 was within ± 6% of the mean value, and whether the measurement was of sufficient length to account for variability in VO2 and VCO2. A minimum of 10 minutes of breath-by-breath data was utilized to obtain the RQ and REE for each patient.
Statistical Analysis
A 2-way analysis of variance was used to estimate the effect of gender and age, in addition to the primary differences between groups. A P value of 0.05 or less was used for determination of statistical significance. Systat (Version 9.0, Systat, Inc) was the statistical software package used for the analyses.
RESULTS
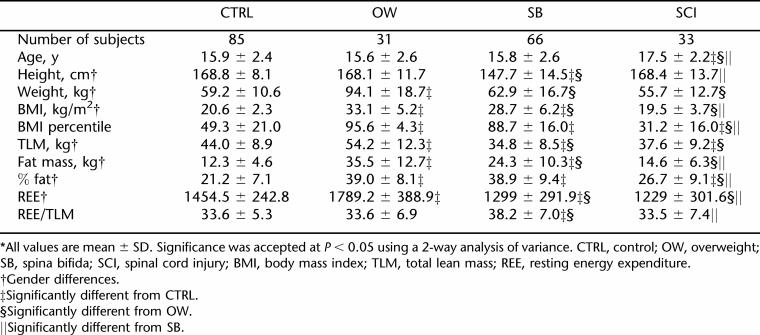
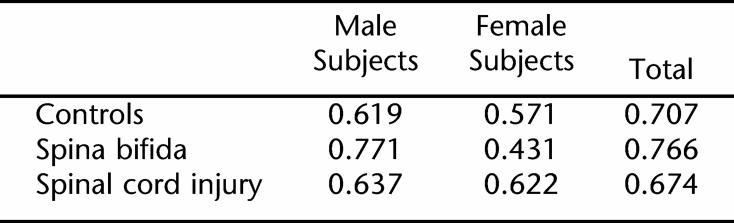
Table 1 describes the anthropometric data for the CTRL, OW, SB, and SCI groups. There were no differences between genders with respect to age, BMI for age, and REE per TLM.
Table 1.
Subject Anthropometric Data*
Height, Weight, and BMI
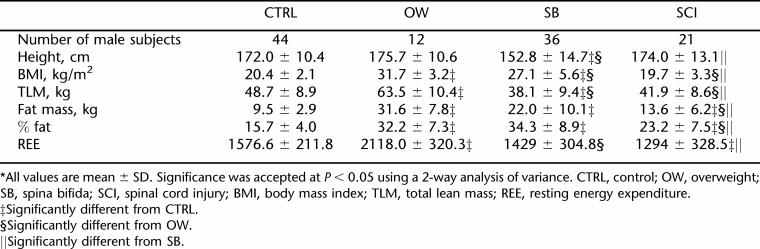
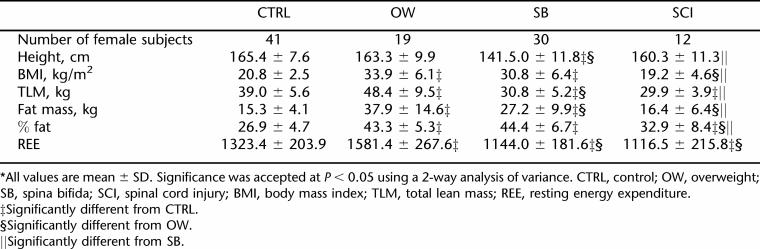
Results from 2-way analysis of variance revealed no significant difference in height between the CTRL and OW groups, but the SB group was significantly shorter than the control, OW, and SCI groups. Male subjects were significantly taller than female subjects in all groups tested (P < 0.05) (Tables 2 and 3). The members of the OW group weighed significantly more than the members of the SB, SCI, and CTRL groups did. The BMI of the OW group was significantly higher than that of the SB group, which in turn was significantly higher than those of both the CTRL group and SCI group. The BMIs of the CTRL and SCI groups were not significantly different from each other.
Table 2.
Gender Characteristics of Male Subjects*
Table 3.
Gender Characteristics of Female Subjects*
Body Composition Measured by DXA
SB subjects had the lowest TLM (34.8 ± 8.5 kg) (P < 0.005) compared to the CTRL (44.0 ± 8.9 kg) and OW (54.2 ± 12.3 kg) groups, but there was no significant difference in TLM between SB and SCI. Although the OW group had significantly higher fat mass than all other groups, there was no significant difference between the percent fat (% fat) of the OW (39 ± 8.0) vs SB groups (38.9 ± 9.4). Gender differences existed in TLM, fat mass, and % fat, as female subjects had less TLM, more fat mass, and higher % fat than their male counterparts in all 4 groups.
Resting Metabolic Rate
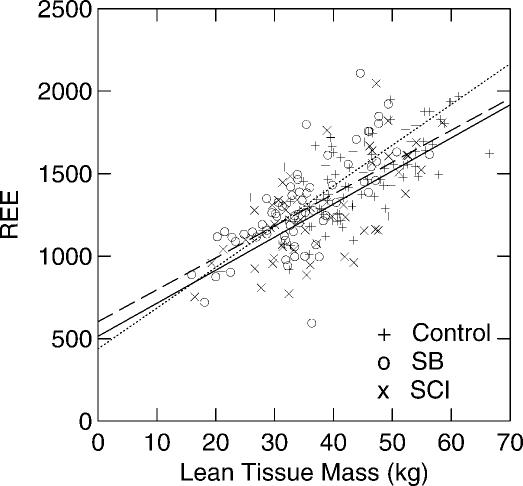
While the SB and SCI groups had significantly lower REE values compared to the CTRL and OW groups (Table 1), when REE was adjusted for kilograms of TLM, there were no differences in REE/TLM ratio among the CTRL, OW, and SCI groups. SB, on the other hand, had significantly higher REE/TLM ratios as compared to the REE/TLM ratios measured in the CTRL, OW, and SCI groups (Table 1). Gender differences existed in REE in all groups, with higher REE in male subjects than female subjects (Tables 2 and 3). There were no significant gender differences when adjusting REE for TLM (Table 1). Figure 1 shows the correlations between REE and TLM for CTRL, OW, SCI, and SB. TLM and REE were more highly correlated in male than in female subjects in all groups (Table 4).
Figure 1. Relationship of the lean tissue mass (LTM, kg) and resting energy expenditure (REE) for SB (o), SCI (x), and control (+) subjects. The dashed line represents the best fit regression for the control group (REE = 19.34 × LTM + 603; r = 0.71). The solid line represents the best fit regression for the SCI group (REE = 20.04 × LTM + 514; r = 0.67). The dotted line represents the best fit regression for the SB group (REE = 25.12 × LTM + 437; r = 0.77).
Table 4.
The Correlation Coefficient (r) for REE and LTM
DISCUSSION
This is the largest study to date evaluating resting energy expenditure in patients aged 11 to 21 years with SCD due to SB and SCI vs an able-bodied population. Our use of DXA as a measure of body composition in this study allowed us to explore the relationship between REE and TLM in both able-bodied young people and those with SB and SCI and varying degrees of adiposity. Our data support previously published reports of lower REE in children and adolescents with SB and SCI, higher REE in children with obesity, decreased TLM in children and adolescents with SCI and SB, and higher fat mass in children and adolescents with SB. However, there are some important differences in our findings from previously reported values. The mean TLM of the SB group in this study was 34.8 kg, which was much greater than 18 to 20 kg reported in the study by Grogan et al (14). In the current study, the mean whole body fat percentage was 38.9%, while Grogan et al reported 53% to 55% whole body fat. The large discrepancies between the body fat percentage reported in the current study compared to the study by Grogan et al. are probably due to methodological differences in body composition measurement (total body potassium vs DXA in this study) as well as the differences in age group studied (5–16 y vs 11–21 y in this study). BMI in the SB group was significantly different from that in CTRL and OW, but the % fat was similar to the OW group, suggesting that BMI may not be an accurate measure of body fat in subjects with SB. In addition, we found a similar BMI in the CTRL and SCI groups, but significantly higher % fat in the SCI group when compared to CTRL. This indicates that BMI may not be a useful anthropometric tool for subjects with SCI as well. The inaccuracy of BMI in patients with SCD due to SB and SCI is probably due to factors associated with difficulty in assessing accurate height because of scoliosis and hip and knee contractures as well as segmental growth retardation of lower limbs. Similar results suggesting that BMI may not be an appropriate screening tool to classify children and adolescents with SCI as obese, overweight, or underweight were previously documented by our group (31).
Our data show similar results to Verga et al (38) when comparing CTRLs to the OW group. Our OW group had significant higher TLM and fat mass probably because lean tissue usually increases concomitantly with extra fat accumulation. Interestingly, out of all the groups examined, the SB group had the lowest TLM but similar % fat when compared to the OW group, which suggests significant relative obesity in this population. Shepherd et al (6) reported that children younger than age 3 to 4 years have body composition similar to the reference values and that excess adipose tissue is acquired with increasing age and decreasing ambulatory activity. Thus, health and fitness interventions consisting of regular physical activity and diet counseling should be started early in the SCD population in order to minimize excess body fat accumulation and maximize relative lean body mass to improve physical fitness and daily functioning.
REE was investigated in the 4 groups of subjects. The results illustrate that REE, expressed in absolute values (kcal/day), was significantly higher in the OW group than in the CTRL, SB, and SCI groups. The absolute REE in our study was the lowest in subjects with SB and SCI as compared to their nondisabled counterparts, with a 10.7% decrease in SB and 15.5% lower in SCI (Figure 1). Similar results on REE has been found in several previous studies (32–34). A study by Spicer et al found REE to be lower than predicted REE, while others found different relationships between REE and predicted REE (30). Conclusions drawn from these studies must be treated cautiously because of the lack of power of the studies due to small sample sizes (n = 6 and n = 19, respectively). There is a gender effect in all groups, as the absolute REEs were significantly higher in male subjects than female subjects, which may be explained by the higher lean tissue mass in males. When REE was adjusted for lean tissue mass, there was no gender effect in all groups.
It has been suggested that the differences in REE between SCD groups and able-bodied groups are related to differences in body composition, specifically total lean mass. Our results partially support this conclusion. The mean REE for OW when adjusted for TLM was not significantly different from that of the CTRLs. However, the SB group had a significantly higher REE/TLM than any of the other 3 groups, including SCI. Verga et al (38) reported similar results with subjects with SCI and concluded that the higher REE observed in the obese control group seemed to be the result of an increased amount of lean tissue that occurs concomitantly with the accumulation of adipose mass (38). Since similarities in body composition exist between the SCI and SB populations, one would expect the relationships between TLM and REE to be similar. A moderate-to-strong correlation between REE and body composition has been documented in SCI patients (31,36,39). Spungen et al (39) and Liusuwan et al (31) found no differences in REE in SCI as compared to CTRL groups when adjusted for TLM. These studies suggest that a reduction in TLM is associated with a reduction in REE in adult and pediatric patients with SCI. Contrary to the presumption, our study showed significant differences in REE when adjusted for TLM in SB as compared to CTRL, OW, and SCI groups. Interestingly, the REE/TLM ratios were remarkably constant in the CTRL, OW, and SCI groups but significantly elevated in the SB group.
It is not known why subjects with SB had significantly higher REE/LTM ratios than the CTRL, OW, and SCI groups. Our results suggest that for patients with SB, a loss of muscle mass may not be the only important factor influencing REE. One would expect an even greater degree of adiposity in the SB group if their REE/TLM ratios were not elevated relative to those without congenital paralysis. Although skeletal muscle and adipose tissue are the 2 largest tissue/organ components in the body, their contribution to REE is smaller than that of other organs. At rest, the majority of the energy expended by the body arises from organs such as the liver, kidneys, heart, and brain, which account for only 5% to 6% of body mass (40). A prediction model for REE based upon the metabolic requirements of tissues and organs is REE = 200 ×liver + 240 ×brain + 440 ×heart + 440 ×kidneys + 13 × skeletal muscle + 4.5 × adipose tissue + 12 × miscellaneous tissues, where REE is in kilocalories per day, individual tissue/organ mass is in kilograms, and the coefficients are the resting metabolic rates of individual tissues and organs in kilocalories per kilogram per day (41). Perhaps there is an upregulation of metabolism in certain tissues and organs of individuals with congenital paralysis relative to able-bodied controls. However, this has yet to be demonstrated and was not examined in the current study.
Factors that have been shown to increase REE/LTM for other diseases include inflammation, increased thyroid status, and increased stress. Subjects with SB and SCI have frequent urinary tract infections (UTIs) that may be responsible for increased inflammation. Chronic infection or hypermetabolic state is unlikely to be the explanation for higher REE/TLM in SB because the SCI population also has recurrent infections such as UTIs. Other factors that contribute to higher REE/TLM ratio in SB may be involved, but the mechanism is unclear to date and merits further study.
CONCLUSIONS
In conclusion, REE per kilogram of TLM in patients age 11 to 21 years with SB was much higher than in SCI, able-bodied obese, and able-bodied nonobese subjects of the same ages. We suggest that obesity in SB is not completely due to decreased REE but may be multifactorial, including a greater relative reduction in physical activity and/or an excessive relative food intake. Thus, early intervention programs including exercise, nutrition, and education to improve overall fitness and prevent obesity would appear to be critical in children and adolescents with SB.
Acknowledgments
Supported in part by Shriners Hospitals for Children Project number 8600: Exercise and Dietary Intervention in Obese Children with Paraparesis due to Spinal Cord Dysfunction; National Institute of Disability and Rehabilitation Research Grant #H133B031118; Rehabilitation Research and Training Center in Neuromuscular Diseases: Enhancing Health, Function, and Quality of Life; National Institute of Child Health and Human Development Grant # RO1 HD35714: Child Mobility: Role of Strength, Body Fat & Energy Cost.
REFERENCES
- Charney EB, Rosenblum M, Finegold D. Linear growth in a population of children with myelomeningocele. Z Kinderchir. 1981;34:415–419. doi: 10.1055/s-2008-1063385. [DOI] [PubMed] [Google Scholar]
- Fiore P, Castagnola E, Palmieri A, et al. Nutritional survey of children and adolescents with myelomeningocele (MMC): overweight associated with reduced energy intake. Eur J Pediatr Surg. 1998;8(Suppl 1):34–36. doi: 10.1055/s-2008-1071250. [DOI] [PubMed] [Google Scholar]
- Hayes-Allen MC. Obesity and short stature in children with myelomeningocele. Dev Med Child Neurol. 1972;27(Suppl):59–64. doi: 10.1111/j.1469-8749.1972.tb09775.x. [DOI] [PubMed] [Google Scholar]
- Mita K, Akataki K, Itoh K, Ono Y, Ishida N, Oki T. Assessment of obesity of children with spina bifida. Dev Med Child Neurol. 1993;35:305–311. doi: 10.1111/j.1469-8749.1993.tb11642.x. [DOI] [PubMed] [Google Scholar]
- Roberts D, Shepherd RW, Shepherd K. Anthropometry and obesity in myelomeningocele. J Paediatr Child Health. 1991;27:83–90. doi: 10.1111/j.1440-1754.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Shepherd K, Roberts D, Golding S, Thomas BJ, Shepherd RW. Body composition in myelomeningocele. Am J Clin Nutr. 1991;53:1–6. doi: 10.1093/ajcn/53.1.1. [DOI] [PubMed] [Google Scholar]
- Rosenblum MF, Finegold DN, Charney EB. Assessment of stature of children with myelomeningocele, and usefulness of arm-span measurement. Dev Med Child Neurol. 1983;25:338–442. doi: 10.1111/j.1469-8749.1983.tb13767.x. [DOI] [PubMed] [Google Scholar]
- Shurtleff DB, Lamers J, Goiney T, Gordon L. Are myelodys-plastic children fat? Anthropometric measures: a preliminary report. Spina Bifida Ther. 1982;4:1–21. [Google Scholar]
- Olle MM, Pivarnik JM, Klish WJ, Morrow JR., Jr. Body-composition of sedentary and physically active spinal-cord injured individuals estimated from total-body electrical-conductivity. Arch Phys Med Rehabil. 1993;74:706–710. doi: 10.1016/0003-9993(93)90030-e. [DOI] [PubMed] [Google Scholar]
- Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury—a retrospective study. Spinal Cord. 2006;44:92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Widman L, Walsh SA, Page P, Abresch RT. Community heart rate monitoring in disabled populations as a measure of physical activity. J Spinal Cord Med. 2004;27(suppl 1):S118–S119. [Google Scholar]
- van den Berg-Emons HJ, Bussmann JB, Meyerink HJ, Roebroeck ME, Stam HJ. Body fat, fitness and level of everyday physical activity in adolescents and young adults with meningomyelocele. J Rehabil Med. 2003;35:271–275. doi: 10.1080/16501970310012400. [DOI] [PubMed] [Google Scholar]
- Widman L, Abresch RT, Styne DM, McDonald CM. Aerobic fitness, upper extremity strength, and body composition in patients aged 11–21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med. 2007;30:S91–S100. doi: 10.1080/10790268.2007.11754611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan CB, Ekvall SM. Body composition of children with myelomeningocele, determined by 40K, urinary creatinine and anthropometric measures. J Am Coll Nutr. 1999;18:316–323. doi: 10.1080/07315724.1999.10718870. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Raza M, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med. 1992;59:163–168. [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy x-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005;29:1346–1352. doi: 10.1038/sj.ijo.0803026. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Policy Statement (Committee on Nutrition) Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- Littlewood RA, Trocki O, Cleghorn G. Measured and predicted total body water in children with myelomenin-gocele. J Paediatr Child Health. 2003;39:278–281. doi: 10.1046/j.1440-1754.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Wilmet E, Ismail AA, Heilporn A, Welraed D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Hanson JA. Body composition by dual-photon absorptiometry and dual-energy x-ray absorptiometry. Basic Life Sci. 1990;55:427–432. doi: 10.1007/978-1-4613-1473-8_60. [DOI] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- Chow YW, Inman C, Pollintine P, et al. Ultrasound bone densitometry and dual energy x-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord. 1996;34:736–741. doi: 10.1038/sc.1996.134. [DOI] [PubMed] [Google Scholar]
- Laskey MA. Dual-energy x-ray absorptiometry and body composition. Nutrition. 1996;12:45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of body-composition techniques against dual-energy x-ray absorptiometry in young children. Am J Clin Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- Gutin B, Litaker M, Islam S, Manos T, Smith C, Treiber F. Body-composition measurement in 9–11-y-old children by dual-energy x-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am J Clin Nutr. 1996;63:287–292. doi: 10.1093/ajcn/63.3.287. [DOI] [PubMed] [Google Scholar]
- Bandini L, Schoeller DA, Fukagawa NK, Wykes L, Dietz WH. Body composition and basal metabolic rate (BMR) in children and adolescents with myelodysplasia. Am J Clin Nutr. 1988;47:776–780. [Google Scholar]
- Littlewood RA, Trocki O, Shepherd RW, Shepherd K, Davies PS. Resting energy expenditure and body composition in children with myelomeningocele. Pediatr Rehabil. 2003;6:31–37. doi: 10.1080/1363849031000097817. [DOI] [PubMed] [Google Scholar]
- Liusuwan A, Widman L, Abresch RT, McDonald CM. Altered body composition affects resting energy expenditure and interpretation of body mass index in children with spinal cord injury. J Spinal Cord Med. 2004;27(Suppl 1):S24–S28. doi: 10.1080/10790268.2004.11753781. [DOI] [PubMed] [Google Scholar]
- Mollinger LA, Spurr GB, el Ghatit AZ, et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66:420–426. [PubMed] [Google Scholar]
- Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma. 1985;25:419–423. [PubMed] [Google Scholar]
- Lee BY, Agarwal N, Corcoran L, Thoden WR, Del Guercio LR. Assessment of nutritional and metabolic status of paraplegics. J Rehabil Res Dev. 1985;22:11–17. doi: 10.1682/jrrd.1985.07.0011. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- Sedlock DA, Laventure SJ. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia. 1990;28:448–454. doi: 10.1038/sc.1990.60. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Lewis RD, Yetman KA, et al. Comparison of body composition and bone mineral measurements from two DXA instruments in young men. Am J Clin Nutr. 1996;64:669–676. doi: 10.1093/ajcn/64.5.669. [DOI] [PubMed] [Google Scholar]
- Verga S, Buscemi S, Caimi G. Resting energy expenditure and body composition in morbidly obese, obese and control subjects. Acta Diabetol. 1994;31:47–51. doi: 10.1007/BF00580761. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Bauman WA, Wang J, Pierson RN., Jr. The relationship between total body potassium and resting energy expenditure in individuals with paraplegia. Arch Phys Med Rehabil. 1993;74:965–968. [PubMed] [Google Scholar]
- Wang Z, Heshka S, Zhang K, Boozer CN, Heymsfield SB. Resting energy expenditure: systematic organization and critique of prediction methods. Obesity Res. 2001;9:331–336. doi: 10.1038/oby.2001.42. [DOI] [PubMed] [Google Scholar]
- Kleiber M. The Fire of Life: An Introduction to Animal Energetics. New York, NY: Wiley; 1961. [Google Scholar]