Abstract
Background/Objective:
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells (MSCs) that can be extracted from adipose tissue and obtained by a less invasive method and in larger quantities compared with bone marrow–derived MSCs. The objective of this study was to harvest ADSCs from piglets and to explore their neuronal differentiation potential.
Methods:
Adipose tissue from piglet facial or abdominal fat was digested with collagenase type XI, followed by filter and centrifugation; the isolated adipose stromal cells were cultured in dishes. MSC markers were measured by flow cytometry; 2 to 5 passage cells were used for in vitro differentiation. Adipogenic, chondrogenic, osteogenic, and neuronal differentiation was induced by incubation of the ADSCs with different induction media.
Results:
ADSCs were easily expanded to beyond 15 passages, maintaining the undifferentiated state and exhibiting MSC characteristics and markers CD29, CD44, and CD90. ADSCs differentiated into other mesodermal cells including adipocytes, chondrocytes, and osteocytes. These cells were induced to differentiate into neuron-like cells as evidenced by neuronal morphology and the presence of neuronal markers including microtubule-associated protein 2, neuronal nuclear antigen, and β-tubulin III.
Conclusions:
ADSCs can be readily obtained from a small amount fat tissue and expanded in culture. Adipose tissue may be an alternative source of stem cell therapy for nervous system injury.
Keywords: Mesenchymal stem cell, Adipose tissue, Neuronal differentiation
INTRODUCTION
Currently, there is no effective treatment for children with central nervous system injury. Stem cell–based therapies hold great promise. Mesenchymal stem cells (MSCs) are found in many peripheral and easily accessible adult tissues and represent an attractive autologous supply of stem cells for therapeutic purposes. MSCs are undifferentiated cells that have the ability to self-renew with a high proliferative capacity and possess a mesodermal differentiation potential (1). These stem cells have been detected in bone marrow, umbilical cord blood, and adipose tissue (2,3). Recent studies have shown that bone marrow stromal cells (BMSCs) may have neuronal differentiated potential (4–6). However, BMSCs must be obtained by bone marrow biopsy, a potentially painful procedure, and the yield from this procedure is relatively low. In addition, the maximal life span of BMSCs decline with increasing age (7–9). Adipose tissue has been identified as an alternative source of multipotent stromal MSCs, which can be obtained by a less invasive method and in larger quantities compared with BMSCs. Adipose-derived stem cells (ADSCs) harvested from piglets have never been reported. The primary goals of this study are to harvest ADSCs from piglets and to explore their neuronal differentiated potential.
METHODS
All animal procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved before implementation by the Institutional Animal Care and Use Committee of Miami University. The animals were housed under conditions approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Tissue sharing of adipose tissue was done from anesthetized healthy infant piglets.
The adipose tissue was obtained from the facial region (the fat pat behind the eyeball) at the time of craniotomy for induction of stroke in infant piglets or from the subcutaneous region of the lower abdominal wall. During this experiment, tissues acquired from 4 different piglets were used. Isolation of ADSCs was accomplished using a modification of published methods (10,11). The tissues were washed with phosphate-buffered saline (PBS) buffer and mechanically dissociated and digested with collagenase type XI (Sigma) for 30 to 60 minutes at 37°C with intermittent shaking. The suspension was filtered through an 80-μm nylon mesh and centrifuged to separate the floating adipocytes from the stromal cells. The preadipocytes in the stromal vascular fraction were plated in T75 flasks at 8,000 to 10,000 cells/cm2 in Dulbecco minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (control media; Gibco BRL, Rockville, MD). Cells were used for in vitro differentiation studies after 2 to 5 passages. Only samples that showed at least 80% confluence were used for these experiments. Unless otherwise noted, all experiments were performed on separate cultures in triplicate.
For phenotype characterization, MSC markers CD29, CD44, and CD90 were measured by flow cytometry using allophycocyanin (APC), phycoerythrin (PE), or fluorescein isothiocynate (FITC) directly conjugated antibodies (BD Biosciences, San Diego, CA). To verify the multipotential differentiation of mesenchymal lineages of ADSCs, cells were subjected to differentiation in conditions known to induce adipogenic, osteogenic, and chondrogenic lineages. For adipogenic differentiation, ADSCs were induced by control media supplemented with 10 ng/mL insulin and 10−7 M dexamethasone for 3 weeks. Adipogenic differentiation was visualized by the presence of intracellular lipid droplets in the phase contrast microscopy and stained by Oil-Red O. To induce osteogenic differentiation, ADSCs were fed with control medium to which was added 10 mM β-glycerophosphate, 0.2 mM ascorbic acid, and 10−7 M dexamethasone for 3 weeks. Mineralization of the extracellular matrix was visualized by staining with Alizarin red S. Chondroblast differentiation was induced by media supplemented with 6.25 μg/mL insulin, 10 ng/mL transforming growth factor (TGF)-β1, and 50 ng ascorbate-2-phosphate in control medium for 3 to 4 weeks. Chondrogenesis was confirmed by glycosaminoglycans staining with 0.1% Safranin O.
To induce neuronal differentiation, ADSCs were exposed to a cocktail of neuron induction medium (NIM) (11,12). ADSCs from passages 2 to 5 were grown to 80% confluence in control media. To initiate neuronal differentiation, the cells were washed with PBS, and NIM was added. NIM consisted of DMEM, with 10 μM forskolin, 200 μM in 0.5% ethanol, 5 mM KCl, 2 mM valproic acid, 1 μM hydrocortisone, and 5 μg/mL insulin. All experiments on ADSCs were performed 5 hours to 3 days after exposure to NIM. Neuronal differentiation was indicated by neuronal morphology and neuron markers expression, including microtubule-associated protein 2 (MAP2) (1:250; Cell Signalling, Danvers, MA), β-tubulin III (1:300; Sigma, St. Louis, MO), neuronal nuclear antigen (NeuN) (1:25; Invitrogen, ZYMED, La Jolla, CA), and glial fibrillary acidic protein (GFAP) (1:500; DAKO, Denmark) measured by immunofluorescence and Western blot. For immunofluorescence, we used antibodies directed against MAP2 (1:250; Cell Signal), β-tubulin III (1:300; Sigma), NeuN (1:25; Invitrogen, ZYMED), and GFAP (1:500; DAKO). Control reactions with the primary antibody omitted were routinely performed. Fluorescein-conjugated anti-rabbit or anti-mouse IgG were used as secondary antibodies (Vector Laboratories, Burlingame, CA). For Western blot, the blot was probed either with anti-MAP2 (1:750), anti-NeuN (1:250), anti-β-tubulin III (1:500), or anti-GFAP (1:1000). Immunoreactive bands were detected using horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG antibodies (Invitrogen) and visualized by enhanced chemiluminescence (Amersham: Piscataway, NJ). Actin served as an internal protein control.
HEK 293 cells and primary culture astrocytes were used to determine whether NIM induces nonspecific morphologic changes. HEK 293 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) and cultured in DMEM containing 10% FBS. Primary cultures of astrocytes were generated from newborn cortex as previously described (13) and maintained in standard medium (DMEM with high glucose; Gibco/BRL) containing 10% FBS. Both 80% to 90% confluence HEK 293 cells and astrocytes were exposed to NIM for 24 hours as ADSCs.
RESULTS
ADSCs appeared as a monolayer of broad, flat cells. As the cells approached 80% confluence, they changed to a more spindle-shaped, fibroblastic morphology. More than 98% of cells expressed MSC markers CD29, CD44, and CD90. They were easily expanded up to 15 passages, maintaining the undifferentiated state as spindle-shaped, fibroblastic morphology, and MSCs markers. ADSCs did not spontaneously differentiate during in vitro culture. After adipogenic induction, ADSC displayed an adipogenic phenotype shown by the accumulation of neutral lipid vacuoles. Chondrogenic differentiation was confirmed by the secretion of cartilage-specific glycosamine. Osteogenic differentiation was detected by an osteogenic phenotype and an increase in calcium deposition (Figure 1). After neuronal induction, ADSCs displayed changes in cellular morphology including shrinkage of cytoplasm, formation of axons, and dendrite-like cytoplasmic projections. Three hours after neuronal induction, the cytoplasm of ADSCs began to retract toward the nucleus, forming contracted cell bodies with extended cytoplasmic extensions. After 20 hours, the cell bodies became increasingly spherical with multiple cell processes, exhibiting a neuronal appearance. The histology changes after neuronal differentiation persisted for up to 6 days. Beyond 7 days, cells began to die, and all cells died within 10 to 14 days of culture in NIM (Figure 2). Before exposure to NIM, ADSCs do not express neuron markers. Twenty-four hours after exposure to NIM, ADSCs stain positive for the following neuronal markers: MAP2, β-tubulin III, and NeuN (Figure 3). Neuronal marker expression after neuronal induction was further confirmed by Western blot (Figure 4). The NeuN bands after neuronal induction were present at molecular weights of approximately 46, 48, 66, and 150 kd. Both immunofluorescence and Western blot showed that ADSCs express low levels of the glial cell marker GFAP under control conditions; 24 hours after neuronal differentiation, ADSCs increased staining of GFAP (Figures 3 and 4). Because one of the compounds in NIM, butylated hydroxyanisole (BHA), is known to be cytotoxic, and because of the rapidity with which ADSCs changed morphology when exposed to NIM, there is the concern that the cellular changes we documented were artifacts of the ingredients of NIM. We therefore tested the ability of NIM to induce similar changes in 2 other mature cell types: HEK 293 cells and primary culture astrocytes from rats. We observed no morphologic changes in HEK 293 cells or astrocytes for up to 48 hours after exposure to NIM.
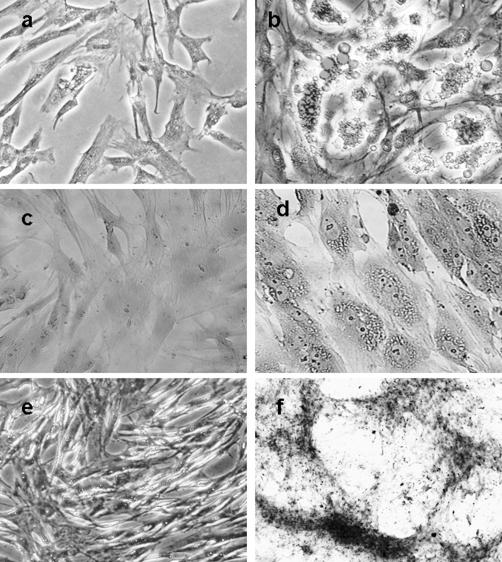
Figure 1. Multilineage differentiation capacity of ADSCs. Adipogenesis was detected by the formation of neutral lipid vacuoles stainable with Oil-Red O, shown in b (a: noninduced control). Chondrogenesis was indicated by glycosaminoglycans with Safranin O staining, shown in d (c: noninduced control). Osteogenesis was shown by the deposition of calcium indicated by Alizarin red stain, shown in f (e: noninduced control) (a–d, magnification, ×200; e and f, magnification, ×100).
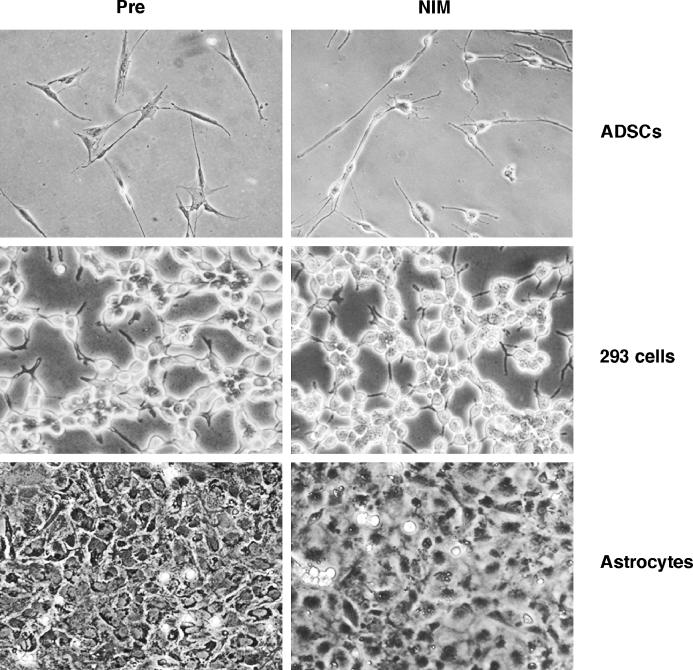
Figure 2. Morphologic changes after neuronal induction of ADSCs and other cells lines. Piglet ADSCs grow as a monolayer of large, flat cells under control conditions in DMEM/10% FBS. Twenty-four hours after NIM exposure, ADSCs showed neuronal morphology as elongated dendritic processes and retracted cell bodies. Both 293 cells and astrocytes failed to show neuronal morphology 24 hours after NIM exposure (magnification, ×200).
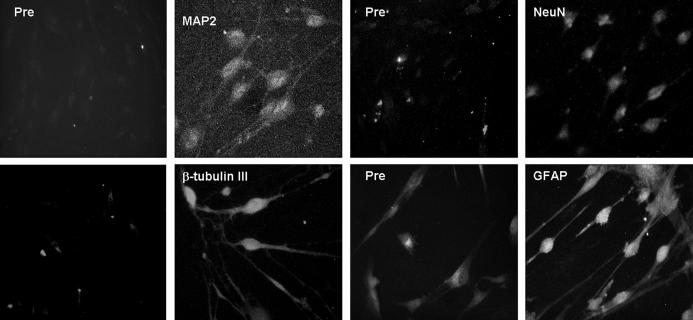
Figure 3. Immunofluorescence of piglet ADSCs after 24 hours of neuronal induction. (Pre) Before neuronal differentiation, ADSCs lack of neuronal markers expression, and they express weak level of glial cell marker-GFAP. Twenty-four hours after NIM, ADSCs stained positive for MAP2, NeuN, and b-tubulin III; also, they increased expression of GFAP (magnification, ×400).
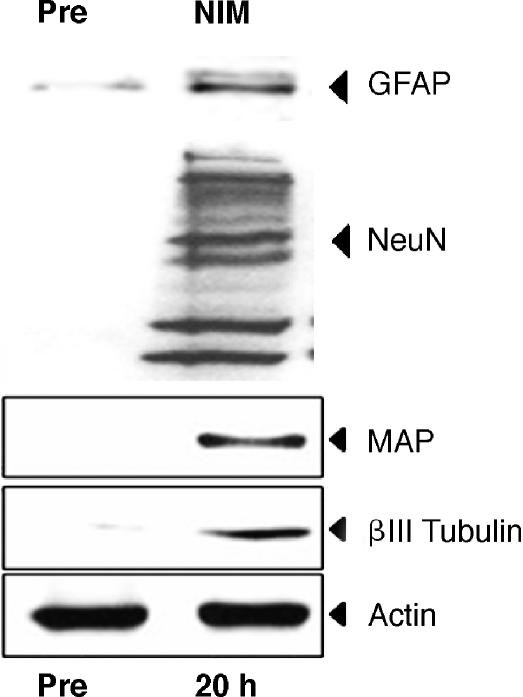
Figure 4. Neuron and glial cells markers expression 24 hours after NIM exposure were further confirmed by Western blot.
DISCUSSION
Until recently, the central nervous system has been generally thought to be incapable of self-repair because mature neurons lack the ability to regenerate and because resident neuronal stem cells are limited in their ability to generate new functional neurons in response to injury. ADSCs are a population of MSCs extracted from adipose tissue. Our results showed that adipose tissue is an alternative source of stem cells that has multiple differentiation potentials and the capacity of neuronal differentiation.
Stem cells have two important characteristics that distinguish them from other types of cells: they are capable of dividing and renewing themselves for extended periods of time, and they can give rise to specialized cell types. Our findings showed that ADSCs can easily be harvested from small amounts of fat tissue and expanded in vitro. They exhibit typical MSC characteristics: fibroblastoid morphology, multipotential capability, and the expression of a typical set of surface markers of MSC. We used ADSCs of 3 to 4 passages for the experiments and observed that more than 98% of cells expressed MSC markers and showed the ability to differentiate into mesodermal lineages, including osteogenic, adipogenic, and chondrogenic. They can be expanded beyond 15 passages, maintaining their undifferentiated state as spindle-shaped, fibroblastic morphology, and their expression of MSCs markers.
Using a cocktail of compounds referred to as NIM, we found that piglet ADSCs can be induced to undergo morphologic and phenotypic changes consistent with developing neuronal cells. They showed a typical neuron-like morphology and expressed the specific neuron markers MAP2, β tubulin III, and NeuN. Our finding that HEK 293 cells and astrocytes failed to show neuronal differentiation or to undergo any morphologic changes after exposure to NIM induction suggests that this phenomenon is not an artifact. In developmental neurogenesis, β tubulin III is expressed in neurons at an early stage of development, and certain isoforms of MAP2 are expressed early during the neuronal differentiation of neural precursor cells (14,15). After neuronal induction, ADSCs showed MAP2 and β tubulin III along the cell body and neurite-like processes, in a similar fashion to developing neurons. ADSCs increase expression of the glial cell marker, GFAP, after neuron induction suggests that these cells may also have glial cell differentiation potential. The results are consistent with published data of BMSCs, which express both neuron and glial cells markers after neuronal induction (16,17), although expression of both neuronal and glial markers by the same cell is difficult to explain.
Recent studies have indicated that both neural induction and neuronal differentiation seem to be connected to the cell cycle control system. Neuronal differentiation induces G1 synchronization and growth arrest, resulting in terminal differentiation. The limited survival of 1 week for differentiated ADSCs may be because of cell cycle arrest and loss of self-renewal capability after differentiation. However, in vivo studies have shown that, after intracerebral transplantation, ADSCs can survive for at least 30 days in the brain and stain positively for neuronal and glial cells markers (18,19). ADSCs may also have immune regulation potential both in vitro and in vivo (20,21), which would prolong their survival in vivo.
CONCLUSION
In summary, these data suggest that ADSCs can be readily obtained from a small amount fat tissue and expanded in culture. These adipose tissue–derived MSCs have the ability to differentiate into multiple cells lines. They can be induced to undergo morphologic and phenotypic changes consistent with neuronal differentiation, and they may have the potential to replace injured neurons after in vivo transplantation. However, although a broad spectrum of phenotypic changes consistent with neuronal differentiation were seen in induced ADSCs, they do not show that these cells were, in fact, functional neurons. Further neurophysiologic studies are needed to confirm whether these differentiated ADSCs function as neurons with respect to the development and maintenance of membrane potentials and action potentials.
Footnotes
This project was supported by NIH grant R21NS044360 (JWK)
REFERENCES
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis. 2001;27:632–636. doi: 10.1006/bcmd.2001.0423. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 1999;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Stenderup K, Justuesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 1999;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy PG, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- Kitani H, Shiurba R, Sakakura T, Tomooka Y. Isolation and characterization of mouse neural precursor cells in primary culture. In Vitro Cell Dev Biol. 1991;27A:615–624. doi: 10.1007/BF02631104. [DOI] [PubMed] [Google Scholar]
- Moody SA, Miller V, Spanos A, Frankfurter A. Developmental expression of a neuron-specific beta-tubulin in frog (Xenopus laevis): a marker for growing axons during the embryonic period. J Comp Neurol. 1996;364:219–230. doi: 10.1002/(SICI)1096-9861(19960108)364:2<219::AID-CNE3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sah DWY, Gage FH. Regulation of voltage- and ligandgated currents in rat hippocampal progenitor cells in vitro. J Neurobiol. 1997;32:95–110. doi: 10.1002/(sici)1097-4695(199701)32:1<95::aid-neu9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Lu J, Moochhala S, Moore XL, et al. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–17. doi: 10.1016/j.neulet.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Kang KS, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15:583–594. doi: 10.1089/scd.2006.15.583. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Pisani D, Deschesne CA. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]