Abstract
Background/Objective:
Children and adolescents who have sustained a spinal cord injury (SCI) are at risk of developing spine deformities and secondary complications that may affect their quality of life. The Shriners Pediatric Instrument for Neuromuscular Scoliosis (SPINS) is a condition-specific instrument that was developed to measure the health-related quality of life (HRQOL) of this patient population. A pilot study was conducted to revise the SPINS and assess comprehensibility.
Methods:
Fourteen children with SCI (ages 6–16 y) from a pediatric hospital were administered either a child version (ages 10–18 y) or a parent version (ages 5–9 y) of the SPINS. Problematic items were identified based on participants' feedback or low statistical variance.
Results:
Ten of 14 (71.6%) respondents understood at least 90% of the items, and 13 out of 14 (92.9%) comprehended more than 80% of relevant items on the SPINS.
Conclusion:
The SPINS has demonstrated comprehensibility. The next step is to measure the validity and reliability of the instrument. The SPINS shows promise as a means of assessing quality of life related to brace effectiveness in children with SCI and neuromuscular scoliosis who primarily use a wheelchair for mobility.
Keywords: Spinal cord injuries, Child, Adolescence, Scoliosis, Rehabilitation, Questionnaire, Bracing, Orthoses, Quality of life, Shriners Hospitals
INTRODUCTION
Children and adolescents with SCI are at risk for developing neuromuscular scoliosis, and the complications associated with a significant spinal deformity (>35 degrees) may affect their quality of life. Most children and adolescents with curves of 20 to 45 degrees receive a back brace to delay curve progression, and some patients begin wearing a brace almost immediately after injury to prevent a curve. If the curve becomes too severe and bracing is ineffective, surgical intervention may be required. The benefits of a condition-specific instrument are becoming more understood, especially as they relate to measurement of health-related quality of life (HRQOL). Many existing functional status and HRQOL questionnaires are not appropriate for children and adolescents with SCI and neuromuscular scoliosis. The majority of the spine questionnaires, such as the Scoliosis Research Society-22 (SRS-22) (1–4) and the Scoliosis Quality of Life Index (SQLI) (5), were designed for adolescents and adults with idiopathic scoliosis. In addition, some of the items within the domains of these questionnaires, especially the physical function domains, are not applicable to the majority of patients with SCI who primarily use a wheelchair. For example, questions about “walking” or “running” are inappropriate for those using wheeled mobility. In addition to the inappropriate items for patients with SCI, few, if any, of these questionnaires have been validated on patients with neuromuscular scoliosis, especially patients with SCI. Finally, some researchers have shown that adult measures should not be used for children and adolescents (6).
Children and adolescents who have sustained a spinal cord injury are at risk of developing spine deformities. The etiology includes muscle weakness or imbalance and residual deformity of the spinal column following fracture or laminectomy. In preadolescents with SCI, Mayfield reported a 96% incidence of scoliosis, with 68% of the patients requiring fusion (7). Lancourt and colleagues reported an overall 82% incidence of scoliosis, with 100% of those injured before the age of 11 years developing scoliosis (8). Dearolf and colleagues supported the earlier literature, with a 96% scoliosis rate in the preadolescent, with 67% requiring fusion (9). In order to assist children and adolescents with spinal deformities, most physicians prescribe bracing when scoliosis progresses to 20 to 45 degrees in skeletally immature children, particularly in those having trouble with sitting posture. Spinal bracing has been shown to be an effective means to assist children with SCI with maintaining sitting balance and posture and can provide stability of the spine for upper extremity activities (10,11). Betz and Mulcahey have investigated results of prophylactic bracing (12). Based on their data, they suggested that bracing of curves less than 20 degrees and less than 1 year from injury in the skeletally immature patient with SCI may prevent or delay surgical intervention over a 5-year follow-up. Two retrospective reviews have reported that prophylactic bracing may prevent severe progression of scoliosis in children with SCI (9,13). Issues for further investigation include the effectiveness of bracing in preventing or delaying surgery and how to measure the impact of bracing vs surgery on the health-related quality of life of children and adolescents with SCI and neuromuscular scoliosis.
The development of a significant spinal deformity (>35 degrees) can lead to pelvic obliquity and asymmetric ischial weight bearing, which may predispose the child to pressure ulcers and impair sitting balance (14,15). Impaired sitting balance may lead to impaired wheelchair mobility, make transfers more difficult, and lead to decreased participation in recreation and leisure activities. In addition, spinal deformity can result in poor upper extremity function. Poor upper extremity use may lead to difficulty performing activities of daily living (ADLs), such as brushing teeth and bathing. Significant spinal deformity may also impact positioning and use of lower extremity orthoses for standing/ambulation. A severe curve can compromise cardiopulmonary function to the point that children may develop pulmonary complications such as pneumonia or respiratory failure, inability to sit upright in a wheelchair for prolonged periods, and/or bowel and bladder dysfunction. A significant spinal deformity can also cause back pain. Such a deformity may also lead to psychological consequences such as causing a child/adolescent to be questioned when compared to other children/adolescents. All of the above-mentioned issues either individually or in combination have the potential to greatly impact the quality of life (QOL) of children and adolescents with SCI and scoliosis.
To date no specific outcomes instrument has been developed to measure the QOL of children with SCI with neuromuscular scoliosis who have undergone treatment involving prophylactic bracing, surgical correction, or both. According to Vitale and colleagues (6), there is more of a focus on outcomes assessment and measurement in adults and less in children. Quite a few questionnaires have been developed for patients with spine deformities, but they have been tested on few, if any, patients with neuromuscular scoliosis and SCI. Wai and colleagues (16) developed a valid and reliable instrument to evaluate physical disability related to scoliosis, but it was tested on patients with spina bifida. Spina bifida and SCI vary with regard to etiology, associated impairments, developmental issues, and level of disability. Bridwell and colleagues (17) developed a questionnaire that assessed patient function, pain, cosmesis, self-image, and the quality of the patient's and caregiver's life. It was tested on 54 patients with neuromuscular scoliosis (33 with Duchenne's muscular dystrophy [DMD] and 21 with spinal muscular atrophy [SMA]). DMD and SMA are progressive neuromuscular disorders, whereas SCI is not progressive. Bridwell's instrument has not been widely utilized in clinical research. Climent and colleagues (5) developed the Quality of Life Profile for Spine Deformities and assessed validity and reliability using a diverse population that included most populations with spine deformities except SCI. The majority of the patients tested were patients with adolescent idiopathic scoliosis (AIS).
Haher and colleagues (18) constructed the SRS-22 as an outcome measure on patients with adolescent idiopathic scoliosis. However, the mean age of patients in a pilot test of the SRS was 25 years (19). In 2006, Asher and colleagues refined the SRS-22 questionnaire, but again, the revisions were based on 111 patients with a mean age of 27.2 years, and only 7 patients had neuromuscular scoliosis (3). In order to address the limitation that adults were utilized to validate the SRS-22 questionnaire, Feise and colleagues tested a modified form of the SRS-22, which they renamed the Scoliosis Quality of Life Index (SQLI), on 10- to 18-year-olds (19). Despite the above, most of the questionnaires used in spinal research have not been tested on children, adolescents, and those with SCI, and there needs to be further research on how valid and reliable these instruments are for the pediatric SCI population.
Children and adolescents who have sustained a SCI do not have the same impairments, functional limitations, and disabilities as patients with spina bifida, DMD, SMA, and adolescent idiopathic scoliosis. Most of the questionnaires that are used for patients with scoliosis do not take into account that some respondents with paraplegia or tetraplegia might be at a disadvantage when answering questions that require a higher level of physical function. For example, the Pediatric Orthopaedic Society of North America (20) developed the Pediatric Outcomes Data Collection Instrument (PODCI) as a functional health outcomes instrument for children and adolescents with a focus on musculoskeletal health. A significant number of questions regarding physical function and sports, as well as transfer and mobility questions, are geared toward ambulatory individuals and not relevant for most patients with neuromuscular scoliosis and SCI. For example, one question asks: “During the last week, has it been easy or hard for you to run short distances?” Another questionnaire, the SRS-2218, contains questions regarding general function pre-and postoperatively, few questions relating to function, and several questions that may not be appropriate for a patient with SCI. For example, one question asks “What is your current level of work/school activity?” The response items include “100% normal, 75% normal, 50% normal, 25% normal, 0% normal.” It may be difficult for someone to decide what “normal” means, especially for a patient with SCI who may interpret “normal” as before his/her spinal cord injury. Asher and colleagues (3) did modify the SRS-22, because of the lack of internal consistency of 2 questions within this domain. However, these 2 questions involved “financial considerations” and “going out with family and friends.” Feise and colleagues addressed these “more social-behavioral” questions by replacing these 2 questions of the SRS-22 in order to design the SQLI as mentioned previously (19). Thus, it seems apparent that a questionnaire with more appropriate physical function questions needs to be developed for certain pediatric populations, especially children and adolescents with SCI and scoliosis.
In addition to assessing physical function, an instrument that could also incorporate other dimensions that constitute a child or adolescent's quality of life would be beneficial. Operationalizing quality of life is challenging, and has been subject to varied interpretation by investigators in the development of QOL instruments. Jang and colleagues (21) state that “there are many published methods for measuring QOL, but there is no consensus on the definition of QOL and the dimensions that should be included in QOL measurement.” Quality of life outcomes measures have been categorized over time into those that measure functional status and those that measure Health-Related Quality of Life (HRQOL). Functional status measures are used to measure activities of daily living (ADLs) and Instrumental ADLs, which are more complex activities that individuals need to be “self-reliant” in the community (22,23). Examples of functional status questionnaires include the Functional Independence Measure (FIM) and the Pediatric Outcomes Data Collection Instrument (PODCI). HRQOL “refers to those components of overall (objective) QOL that center upon or are directly and indirectly affected by health, disease, disorder, and injury: signs, symptoms, treatment side effects, physical, cognitive, emotional, and social functioning, etc.” (25). Examples of HRQOL questionnaires include the Spina Bifida Questionnaire, the SRS-22 (revised), Bridwell's neuromuscular scoliosis questionnaire, and the Medical Outcomes Study Short Form 36 (SF-36).
Even though these functional status and HRQOL measures exist, “many QOL instruments used in SCI research have not been validated for this group (SCI), or have questionable assumptions, and clinical applications of QOL measures still have many problems” (25). For example, some authors consider the SF-36 to be one of the most commonly used generic QOL measures (26,27). Forchheimer and colleagues (27) and a few others have done some initial validity testing of the SF-36 with adults with SCI and have found it to be valid but state that more extensive research is needed. In addition, there is limited research on the validity and other psychometric properties of some functional status and HRQOL questionnaires, including the SF-36, when used with children and adolescents. Some researchers have found that the SF-36 was not appropriate for pediatric patients, even though it has been validated in adults (6,26,27).
Dijkers (29) believes that functional status and HRQOL measures do not represent the individual, and that “individualization” is important in future assessment tools. Dijkers continues to state that future assessment tools should focus on allowing an individual to choose the domains that constitute his/her quality of life and how they rate the responses in terms of satisfaction and importance. According to Dijkers (29), this “individualization” would be beneficial, because “disability does not in and by itself result in diminished QOL.” This is in agreement with Davis and colleagues (30), who feel that there is a lack of research to show that a child's perception of his or her life correlates with his/her ability to perform various tasks/activities. It is becoming more important to use pediatric patient-focused measures in order to evaluate outcomes, especially involving orthopedic interventions, such as surgery (28,31).
Based on the above, it was deemed important to have an outcomes tool that would be able to apply these concepts of “individualization” and “patient-focused” concepts and measure the HRQOL of children with SCI and scoliosis, especially if patients in this population were required to wear braces for their scoliosis or eventually require fusion.
A condition-specific outcomes instrument, designed to have content validity in the assessment of HRQOL of children and adolescents with SCI and neuromuscular scoliosis who primarily use a wheelchair, either manual and/or power, for mobility was developed. The purpose of this manuscript is to introduce the Shriners Pediatric Instrument for Neuromuscular Scoliosis (SPINS) and present pilot data, including comprehensibility and scoring of the instrument.
METHODS
Questionnaire Development
The Shriners Pediatric Instrument for Neuromuscular Scoliosis (SPINS) (originally titled the Paralytic Spine Deformity Outcomes Questionnaire [PSDOQ]) was developed with the ultimate goal of measuring the impact of bracing and/or surgery on the HRQOL of children with SCI and neuromuscular scoliosis. The development of the SPINS began with the identification of the following related domains by a physiatrist with several years of clinical experience working with children with neuro-muscular conditions: sitting balance, activities of daily living (ADLs)/self-care, bowel and bladder management, mobility, sports/recreation/leisure, pain, pulmonary, self-esteem/self-concept, cosmesis, skin integrity, and surgical intervention.
The Delphi technique was used to construct the items within each domain. The team of professionals involved in this process included the physiatrist, a pediatrician, an orthopedic spine surgeon, a rehabilitation therapist, a psychologist, and a biomechanical engineer who work with children with disabilities, especially with SCI. The team of healthcare professionals developed the questionnaire during 3 separate in-person meetings and several conference calls; the professionals reviewed and edited drafts of the instrument and made revisions by consensus. A child/adolescent version for ages 10 to 18 years and a parent version for children 5 to 9 years were developed.
The pilot study version of the SPINS consisted of 108 items within 6 sections. Section A included questions related to the ability to perform certain tasks; Section B measured levels of satisfaction with one's aptitude and Section C the perceived importance of the tasks of Section A. Section D contained items assessing levels of pain, respiratory issues, self-esteem/self-concept, cosmesis, and pressure sores. Section E contained items related to number of hours wearing a spinal orthosis, attitudes towards wearing the spinal orthosis, and perceived helpfulness of the orthosis when engaging in specified activities. Section F assessed outcomes pertinent to patients who had undergone surgery for their scoliosis.
Pilot Study
The objectives of the pilot study were to assess and improve the comprehensibility of the SPINS. Face validity of the items was determined by asking a convenience sample of 14 children, recruited from inpatients and outpatients at Shriners Hospitals for Children Philadelphia, to identify in their own words the purpose of each question. Modifications and revisions to the SPINS were subsequently conducted based on subjects' feedback and measures of the statistical variance for each item.
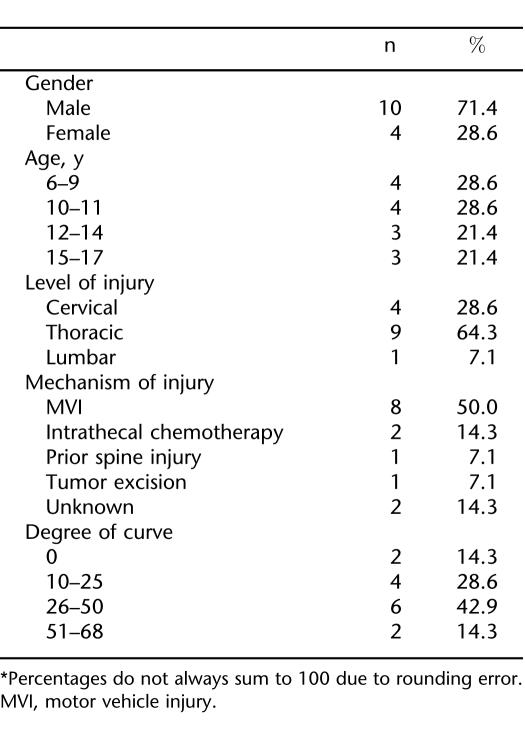
Table 1 presents the gender, age, level, and mechanism of injury, and degree of spinal curvature for the sample. Level of injury ranged from C5 to L1; 11 children had paraplegia and 3 had tetraplegia. Mechanism of injury included motor vehicle injury (n = 8), intrathecal chemotherapy (n =2), prior spine surgery (n = 1), tumor excision (n =1), and unknown (n =2). Scoliotic curves ranged from less than 10 degrees to 68 degrees.
Table 1.
Demographics and Clinical Characteristics of Participants*
Ten individuals were administered the child/adolescent version, and 4 parents completed the parent version of the SPINS. Twelve were prescribed a brace for their scoliosis, so they completed Section E. None of the subjects had undergone surgical intervention, so Section F was not tested in the pilot study.
Administration
Eight of the 14 pilot-test respondents were administered the SPINS via the “cognitive laboratory interview,” a process recommended for the early stages of questionnaire development (32). Per this method, the interviewer read each question aloud to the child/adolescent and then asked:
What is this question asking you?
Are there any words that you do not understand?
Items that the respondents did not fully understand were noted; suggestions were elicited as to how to improve the question.
Subsequently, 6 respondents completed the SPINS by self-administration and were asked to mark an “X” next to the items they did not fully understand. Afterward, suggestions were elicited for modifying the noted items.
Data Analysis
The statistical variance was calculated for the 105 categorical questions. Variance was not calculated on the 3 noncategorical questions. Items with variance less than 0.10 (the criterion selected for unacceptable variation) were eliminated. Elimination of 1 item in Section A, B, or C necessitated the omission of its 2 related measures. Items identified as difficult to understand by 4 or more respondents were modified or deleted as necessary.
Scoring
Scoring of the SPINS requires calculating the mean for the categorical items within each section. The lowest possible value for each item is then subtracted from the mean, divided by the highest possible value for each item minus the lowest possible value, then multiplied by 100. The theoretical range of scores for each section is 0 to 100; higher scores represent better quality of life and outcomes.
RESULTS
Comprehensibility
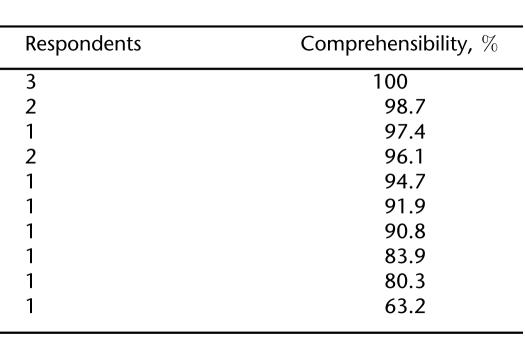
Twenty-two items of the SPINS were deleted and 34 were modified based on the variance less than 0.10 criterion and feedback from the children and parent participants of the pilot study. Comprehensibility was calculated based on the remaining 76 items, except for 3 participants who did not wear a brace and thus did not complete the 14 items of Section E.
The comprehensibility findings indicate that 71.6% (10/14) of respondents understood at least 90% of the items and 92.9% (13/14) comprehended more than 80% of relevant items on the SPINS (Table 2). These data likely represent conservative estimates of comprehensibility as they do not take into account the modifications made to the SPINS subsequent to the pilot study.
Table 2.
Comprehensibility of SPINS
Scores
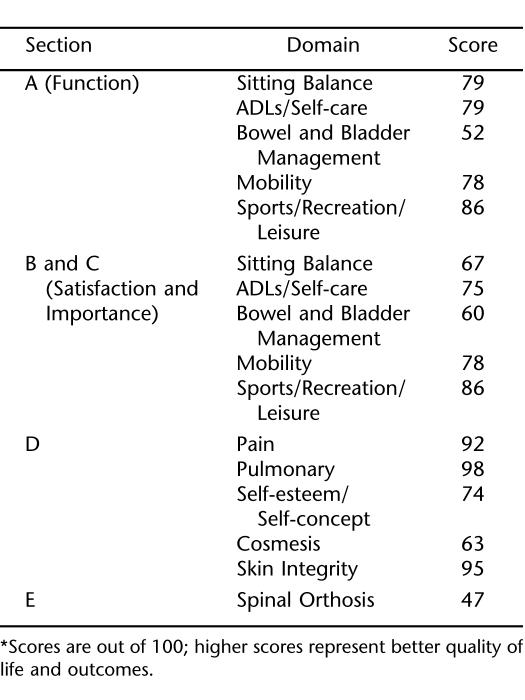
Section A contained questions regarding “function,” defined as the ability to perform certain tasks or activities. The following were the average scores within each domain for all respondents: sitting balance, 79; ADLs/self-care, 79; bowel and bladder management, 52; mobility, 78; and sports/recreation/leisure, 86. For Section B (satisfaction with one's aptitude), the average score for each domain was weighted by the average score for the related domain from Section C (perceived importance) in order to calculate the score out of 100. This weighting was performed so that a respondent's level of importance was considered when calculating satisfaction scores. The following were the average scores for Section B for all respondents: sitting balance, 67; ADLs/self-care, 75; bowel and bladder management, 60; mobility, 70; and sports/leisure/recreation, 79. Section D average scores by all respondents for the following domains were pain, 92; pulmonary, 98; self-esteem/self-concept, 74; cosmesis, 63; and skin integrity, 95. The average score for Section E regarding the effectiveness of a spinal orthosis was 47 (Table 3).
Table 3.
SPINS Scores*
SPINS (Final Items and Response Options)
Section A consists of 19 items that begin by asking, “How would you describe your ability to…” and end by requesting a response based on the “previous week.” Response options are: “Very easy,” “Somewhat easy,” “Somewhat difficult,” “Very difficult, but you could still do it,” and “You couldn't do it at all.”
Four items relate to sitting balance (“reach forward to pick something off the floor with your hand(s) while in your wheelchair”; “reach for something at eye level while in your wheelchair”; “sit on your bed without using your arms or legs for support”; and “reach for something above your head while in your wheelchair”). Three items involve activities of daily living (ADLs) and self-care (“comb your hair”; “put on a shirt”; and “put on pants/skirt”). Two items assess ability to “complete your catheterization” and “complete your bowel program.” Three items measure transfer abilities (“transfer on or off your bed”; “transfer from the wheelchair to the floor”; and “transfer from the floor into the wheelchair”). Ease of wheelchair mobility consists of 3 items: “propel your wheelchair for a long distance (for example, 3 blocks)”; “propel your wheelchair up a ramp in your community”; and “propel your wheelchair outside on uneven surfaces (for example, grass).” One item assesses participation in leisure activities (“such as reading or using the computer”); one item assesses participation in recreational activities/sports with other children (“for example, wheelchair basketball, hand cycling, etc.”); and another item taps into ability to see friends outside of school. Finally, an upright positioning/mobility question reads, “How would you describe your ability to stand/walk using any assistive devices/braces (using any combination of a stander, walker, crutches, leg braces, back brace) as needed during the last week?”
The items of Sections B are presented in the same order as in Section A. The items each begin with, “How satisfied are you with your ability to…” and end with requesting a response based on the “previous week.” Response options to each item in Section B measure levels of satisfaction: “Very satisfied,” “Somewhat satisfied,” “Somewhat dissatisfied,” and “Very dissatisfied.” Section C includes the same items, but response options were: “Extremely important,” “Very important,” “Somewhat important,” and “Not important at all.” Responses to 2 of the 5 items in Section D included: “No pain,” “A little pain,” and “A lot of pain.” These items require respondents to quantify their level of pain “with activity” and “pain that woke you from sleep at night” during the “previous week.” Two items address cosmesis, and the responses include “Same,” “A little different,” and “A lot different.” The items ask “How did your back look compared to other children your age” and “Did you feel that other children and adults thought your back looked the same or different compared to other children your age.” The responses to the last item include “Zero,” “One,” “Two,” “More than three.” This item asks about the number of pressure sores (“on your buttock/legs”) during the “previous year.”
Section E contains items that ask about the spinal orthosis if the child was prescribed to wear one by his/her physician. Two items (noncategorical) ask respondents to identify the number of hours per day they wear the back brace and the number of hours per day the physician prescribed them to wear the brace. The final non-categorical item asks about back pain: “During the last week, how much pain did you have from wearing the brace?” The response items to this question are, “No back pain,” “A little back pain,” and “A lot of back pain.” Another item has the respondents answer “yes” or “no” to the question, “If you were given the choice to wear the brace or not, would you decide to stop wearing the brace?” The responses for the remaining 10 items in this section include “Not at all,” “A little,” “A lot.” Each begins by asking “Does wearing your brace help with….” Each of these items had a different ending. The first five endings were: “sitting balance,” “breathing at rest,” “transfers,” “dressing your upper body,” “dressing your lower body.” The last 5 items include: “propelling your wheelchair,” “the way your back looks,” “completing your catheterization program,” “participation in leisure/recreational activities,” and “completing your bowel program.”
The 10 items in Section F are for those who had surgery for scoliosis during the past 6 months. Each question begins, “Since surgery…” Items ask if surgery had “Greatly improved,” “Improved,” “Not changed,” “Worsened,” or “Greatly worsened” the following: “pain/discomfort,” “the way my back looks,” “breathing,” “sitting balance out of the wheelchair,” “ability to perform catheterization,” “ability to perform bowel care,” “ability to participate in leisure/recreational activities/sports,” “ability to perform daily care,” “ability to transfer from bed to wheelchair,” and “quality of life.”
DISCUSSION
The completion of this pilot study of the SPINS served as the initial step to developing a condition-specific outcomes instrument to measure the HRQOL of children and adolescents with SCI and neuromuscular scoliosis who primarily use a wheelchair for mobility. The comprehensibility data of this study ensured that the items on the questionnaire had a certain level of discriminatory ability and understanding. It is anticipated that a higher percentage of respondents will have a greater understanding of answering the items on the SPINS based on the 34 items that were subsequently modified. Items in the SPINS assess the following domains: sitting balance, activities of daily living (ADLs)/self-care, bowel and bladder management, functional mobility, sports/recreation/leisure, pain, pulmonary, cosmesis, skin integrity, and surgical intervention.
It would be beneficial to use this instrument to further investigate how clinically important spine bracing is to preventing or delaying neuromuscular scoliosis, especially if children and parents think it is more of a burden to a child's quality of life than a benefit.
There were some limitations to this pilot work with the SPINS. First, the sample may not have fully represented the larger population of children and adolescents with SCI and neuromuscular scoliosis, especially those who require surgical intervention for their scoliosis. It would be beneficial to have a much larger sample size from multiple pediatric healthcare facilities that evaluate and treat children and adolescents with SCI and neuromuscular scoliosis. Another limitation was that the sample was one of convenience. It would be beneficial to investigate the SPINS impact on different subgroups within the same sample size. This would provide further evidence to determine whether this condition-specific HRQOL instrument can discriminate among children and adolescents with different severity of spinal curves or levels of injury. Another limitation was that the last section of the SPINS (Section F) was not formally tested along with the other sections due to time restraints and limited number of children with SCI requiring surgical intervention at the time of the pilot work. A final limitation was that scores were not interpreted due to the small convenience sample, but the scoring of the SPINS was solidified based on this pilot work.
Further development of the SPINS is required, especially since it is important for a questionnaire to demonstrate validity, reliability, and responsiveness to change (31).
Footnotes
Funded by Shriners Hospitals Clinical Outcomes Studies Advisory Board grant #9155.
REFERENCES
- Asher M, Lai SM, Burton DC. Further development and validation of the scoliosis research society (SRS) outcomes instrument. Spine. 2000;25:2381–2386. doi: 10.1097/00007632-200009150-00018. [DOI] [PubMed] [Google Scholar]
- Asher M, Lai SM, Burton D. The reliability and concurrent validity of the scoliosis research society-22 patient questionnaire for idiopathic scoliosis. Spine. 2003;28:63–69. doi: 10.1097/00007632-200301010-00015. [DOI] [PubMed] [Google Scholar]
- Asher M, Lai S, Glattes R, et al. Refinement of the SRS-22 health-related quality of life questionnaire function domain. Spine. 2006;31:593–597. doi: 10.1097/01.brs.0000201331.50597.ea. [DOI] [PubMed] [Google Scholar]
- Asher M, Lai SM, Burton D. Scoliosis research society-22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28:70–73. doi: 10.1097/00007632-200301010-00016. [DOI] [PubMed] [Google Scholar]
- Climent JM, Reig A, Sanchez J, et al. Construction and validation of a specific quality of life instrument for adolescents with spine deformities. Spine. 1995;20:2006–2011. doi: 10.1097/00007632-199509150-00011. [DOI] [PubMed] [Google Scholar]
- Vitale MG, Levy DE, Johnson MJ, et al. Assessment of quality of life in adolescent patients with orthopaedic problems: are adult measures appropriate? J Pediatr Orthop. 2001;21:622–628. [PubMed] [Google Scholar]
- Mayfield JK. Severe spine deformity in myelodysplasia and sacral agenesis: an aggressive surgical approach. Spine. 1981;6:498–509. doi: 10.1097/00007632-198109000-00013. [DOI] [PubMed] [Google Scholar]
- Lancourt JE, Dickson JH, Carter RE. Paralytic spine deformity following traumatic SCI in children and adolescents. J Bone Joint Surg Am. 1981;63:47–53. [PubMed] [Google Scholar]
- Dearolf WW, Betz RR, Vogel LC, et al. Scoliosis in pediatric SCI patients. J Pediatr Orthop. 1990;10:214–218. [PubMed] [Google Scholar]
- Brown JC, Swank SM, Matta J, et al. Late spinal deformity in quadriplegic children and adolescents. J Pediatr Orthop. 1984;4:456–461. doi: 10.1097/01241398-198408000-00013. [DOI] [PubMed] [Google Scholar]
- Shakhazizian KA, Massagli T, Southard TL. Spinal cord injury. In: Campbell SK, Vander Linden D, Palisano R, editors. Physical Therapy for Children. Philadelphia, PA: Saunders; 2001. pp. 571–596. [Google Scholar]
- Betz RR, Mulcahey MJ. Spinal cord injury rehabilitation. In: Weinstein SL, editor. The Pediatric Spine: Principles and Practice. New York: Raven Press; 1994. [Google Scholar]
- Mehta S, Betz RR, Mulcahey MJ, et al. Effect of bracing on paralytic scoliosis secondary to spinal cord injury. J Spinal Cord Med. 2004;27(suppl):S88–S92. doi: 10.1080/10790268.2004.11753448. [DOI] [PubMed] [Google Scholar]
- Drummond D, Breed A, Narechania R. Relationship of spine deformity and pelvic obliquity on sitting pressure distributions and decubitus ulceration. J Pediatr Orthop. 1985;5:396–402. doi: 10.1097/01241398-198507000-00002. [DOI] [PubMed] [Google Scholar]
- Kilfoyle RM, Foley JJ, Norton PL. Spine and pelvic deformity in childhood and adolescent paraplegia: a study of 104 cases. J Bone Joint Surg Am. 1965;47:659–682. [PubMed] [Google Scholar]
- Wai EK, Owen J, Fehlings D, et al. Assessing physical disability in children with spina bifida and scoliosis. J Pediatr Orthop. 2000;20:765–769. doi: 10.1097/00004694-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Bridwell KH, Baldus C, Iffrig TM, et al. Process measures and patient/parent evaluation of surgical management of spinal deformities in patients with progressive flaccid neuromuscular scoliosis (Duchenne's muscular dystrophy and spinal muscular atrophy) Spine. 1999;24:1300–1309. doi: 10.1097/00007632-199907010-00006. [DOI] [PubMed] [Google Scholar]
- Haher TR, Gorup JM, Shin TM, et al. Results of the scoliosis research society instrument for evaluation of surgical outcomes in adolescent idiopathic scoliosis. Spine. 1999;14:1435–1440. doi: 10.1097/00007632-199907150-00008. [DOI] [PubMed] [Google Scholar]
- Feise R, Donaldson S, Crowther E, et al. Construction and validation of the scoliosis quality of life index in adolescent idiopathic scoliosis. Spine. 2005;30:1310–1315. doi: 10.1097/01.brs.0000163885.12834.ca. [DOI] [PubMed] [Google Scholar]
- Daltroy LH, Liang MH, Fossel AH, et al. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. J Pediatr Orthop. 1998;18:561–571. doi: 10.1097/00004694-199809000-00001. [DOI] [PubMed] [Google Scholar]
- Jang Y, Ching-Lin H, Wang YH, et al. A validity study of the WHOQOL-BREF assessment in persons with traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1890–1895. doi: 10.1016/j.apmr.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Allan RM. Health-related quality of life outcomes measures. Arch Phys Med Rehabil. 2000;81(Suppl 2):S30–S45. doi: 10.1053/apmr.2000.20621. [DOI] [PubMed] [Google Scholar]
- Meyers A, Andresen EM, Hagglund KJ. A model of outcomes research: spinal cord injury. Arch Phys Med Rehabil. 2000;81(Suppl 2):S81–S90. doi: 10.1053/apmr.2000.20629. [DOI] [PubMed] [Google Scholar]
- Dijkers M. Quality of life after spinal cord injury: a meta analysis of the effects of disablement components. Spinal Cord. 1997;35:829–840. doi: 10.1038/sj.sc.3100571. [DOI] [PubMed] [Google Scholar]
- Dijkers M. Quality of life of individuals with spinal cord injury: a review of conceptualization, measurement, and research findings. J Rehabil Res Dev. 2005;42(3 Suppl 1):87–110. doi: 10.1682/jrrd.2004.08.0100. [DOI] [PubMed] [Google Scholar]
- Lai SM, Asher M, Burton D. Estimating SRS-22 quality of life measures with SF-36: application in idiopathic scoliosis. Spine. 2006;31:473–478. doi: 10.1097/01.brs.0000200049.94329.f4. [DOI] [PubMed] [Google Scholar]
- Forchheimer M, McAweeney M, Tate DG. Use of the SF-36 among persons with spinal cord injury. Am J Phys Med Rehabil. 2004;83:390–395. doi: 10.1097/01.phm.0000124441.78275.c9. [DOI] [PubMed] [Google Scholar]
- Furlong W, Burr RD, Feeny D, et al. Patient-focused measures of functional health status and health-related quality of life in pediatric orthopedics: a case study in measurement selection. Health Qual Life Outcomes. 2005;3:3. doi: 10.1186/1477-7525-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers M. Individualization in quality of life measurement: instruments and approaches. Arch Phys Med Rehabil. 2003;84(Suppl 2):S3–S12. doi: 10.1053/apmr.2003.50241. [DOI] [PubMed] [Google Scholar]
- Davis E, Waters E, Mackinnon A, et al. Paediatric quality of life instruments: a review of the impact of the conceptual framework on outcomes. Dev Med Child Neurol. 2006;48:311–318. doi: 10.1017/S0012162206000673. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler FJ. Survey Research Methods. 3rd ed. Thousand Oaks, CA: Sage; 2001. [Google Scholar]