Abstract
Background/Objective:
Because hydronephrosis and reflux are reversible, we believe cortical loss represents true renal deterioration in children with spinal dysraphism. Our goal was to better define risk factors for cortical loss.
Methods:
After institutional review board approval, we reviewed the medical records of 272 children with spinal dysraphism. The following factors were evaluated: age, sex, renal and bladder imaging, urodynamic parameters, medications, catheterization program, continence, infections, and surgical history. Renal cortical loss was defined by scarring or a differential function greater than 15% using a nuclear scan. Univariate and multivariate logistic regression models were fitted to test the associations of specific variables with cortical loss.
Results:
Renal cortical loss was found in 41% of children with high-grade reflux vs 2% of children without reflux. Univariate analysis showed only high-grade reflux and female sex to be independent risk factors. Controlling for age and sex, reflux and initiation of catheterization after 1 year of age are significant risk factors. High bladder pressure and hydronephrosis in the absence of reflux were not associated with cortical loss. Multivariate analysis showed that girls with reflux have a 55-fold increased risk of cortical loss.
Conclusion:
By limiting the definition of renal deterioration to cortical loss, we identified relevant risk factors: reflux, female sex, and delayed initiation of clean intermittent catheterization. We have also discounted other suspected risk factors: hydronephrosis and elevated bladder pressure. Rather than continuing our focus on hydronephrosis and urodynamics, we believe more research and management debate should be afforded to females with reflux.
Keywords: Spinal dysraphism, Kidney, Vesicoureteral reflux, Bladder, Urodynamics, Hydronephrosis, Spina bifida, Catheterization, Outcomes research, Myelodysplasia
INTRODUCTION
The primary focus of urologic management for children with spinal dysraphism is preservation of renal function. Patients with spinal dysraphism with reflux have been shown to be at greater risk of upper tract deterioration than those without reflux (1,2). Although the term renal “deterioration” in the literature has many meanings, such as hydronephrosis, infection, cortical loss, and reflux, only renal cortical loss is irreversible. Although the lost tissue can not regenerate, this does not imply impaired renal function in all cases. With the common use of early clean intermittent catheterization (CIC), chronic hydro-nephrosis has fortunately become much less common (3–6).
Serial urodynamics (UDS) and renal sonography have been used to follow patients with spinal dysraphism. Although UDS has the ability to predict patients at risk for hydronephrosis and reflux (2,7–9), urodynamics does not prognosticate renal cortical loss. The authors believe that too much emphasis has been placed on hydronephrosis. Based on our clinical experience, we hypothesized that there were more relevant risk factors. With the manifestation of these factors, we hope to improve the clinical management of these children and change the focus of related research.
METHODS
After institutional review board approval, we reviewed the charts of 272 patients with spinal dysraphism who were receiving care at Shiners Hospital of Northern California from September 1985 through June 2003. All patients were seen and followed in a comprehensive, multidisciplinary spinal dysraphism clinic. The average follow-up period was 4.1 years (range, 0.2–15.5 years). All patients were evaluated initially with videourodynamics and renal sonography.
A retrospective chart review of all 272 patients was performed, and data were collected and stored in a commercially available spreadsheet. A multitude of factors were evaluated including patient age, sex, renal and bladder images, medications, initiation of a CIC program, continence, urinary tract infections (UTIs), and surgical history. Reflux of grade 3 or higher (dilating) was considered high grade. CIC instituted before 1 year of age was considered “early.”
Renal cortical loss was strictly defined by evidence of scarring on renal ultrasound or renal scan, a differential function greater than 15% on nuclear scintigraphy, or a difference in renal size of greater than 15% on renal ultrasound compared with the contralateral kidney. Recurrent infections were defined by greater than 2 symptomatic infections per year (fever, pain, or foul odor) requiring antibiotic treatment. Degree of continence was based on a subjective assessment by the patient or family and recorded as dry, occasional leakage, or wet.
Repeat imaging of the upper urinary tract with renal ultrasound was obtained every 6 to 12 months depending on age and risk factors. Renal scans were obtained initially if patients presented with evidence of upper tract changes on ultrasound or cystourethrography, developed upper tract changes, or had recurrent infections. Videourodynamics were performed on all patients. The results from the first urodynamic study and the last were used to compare within and between the groups of patients. Parameters including capacity (based on the standardized volume for age), end-fill pressure, compliance, and reflux were recorded.
Incidence, not prevalence, of risk factors was evaluated. Thus, any history of reflux, UTI, hydronephrosis, incontinence, or elevated bladder pressure was recorded. Many patients were referred at later ages. Some patients did not have complete or adequate data for complete analysis (ie, only 220 of 272 had interpretable upper tract imaging). Statistical analyses began with descriptive statistics of each variable. Univariate logistic regression models were fitted to test the associations of specific variables with renal deterioration. Multivariate logistic regression models were also fitted, which took into account missing data points. All analyses were carried out in SAS Version 8.2 (SAS, Cary, NC), and all tests were 2-sided at level 0.05.
RESULTS
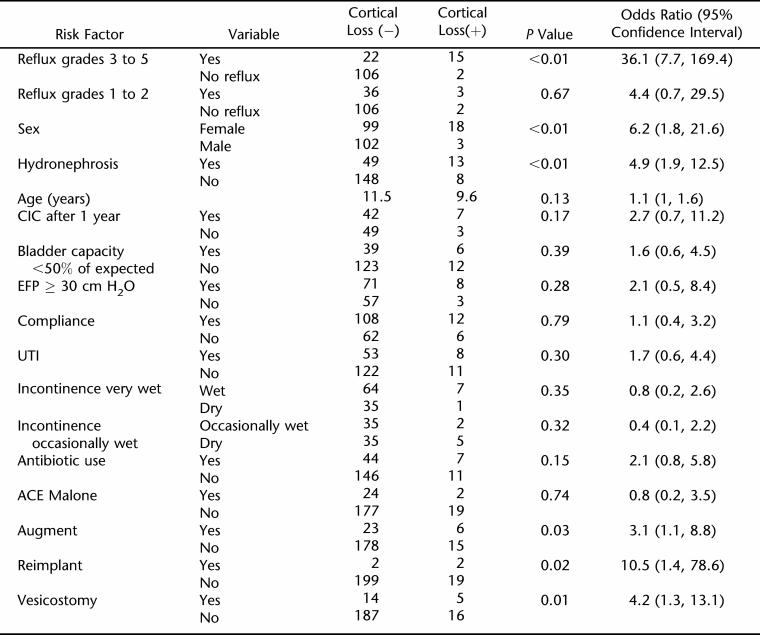
Of the 272 children evaluated, 199 had adequate bladder imaging data, and 45% were found to have a history of reflux during their lifetime. Of 220 patients with interpretable upper tract imaging, 21 (10%) had evidence of cortical loss by our strict definition. Eighteen of 21 patients had greater than 15% difference in renal function by nuclear scintigraphy. Two patients had bilateral cortical scars by sonography and nuclear imaging without substantial functional difference between the right and left kidney. One child had renal failure. Renal cortical loss was found in 17% of children with reflux vs 2% of children without reflux. Of the 37 children with high-grade reflux, 15 (41%) had evidence of cortical loss. Most children with cortical loss were girls (86%; Table 1).
Table 1.
Univariate Associations With Cortical Loss
Univariate Logistic Regression
The variables sex, reflux (both continuous and dichotomized), hydronephrosis, augment, reimplant, and vesicostomy were found to be statistically significant in the univariate models (Table 1). After controlling for age and sex, CIC became significant, and all others remained the same or had no meaningful change in significance.
Multivariate Logistic Regression
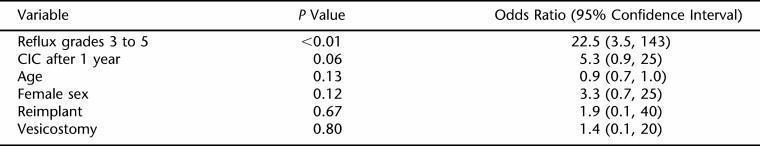
After the noncorrelated significant variables were put in a multivariate model, high-grade reflux remained significant (P < 0.01), and delayed CIC almost became significant (P = 0.06; Table 2). No other variables became significant. Of interest, hydronephrosis without reflux was not associated with cortical loss.
Table 2.
Multivariate Associations With Cortical Loss
In the multivariate logistic regression model, being a girl and having high-grade reflux contribute independently to the risk of scarring. This finding suggests that the group of female patients with high-grade reflux may be at particularly high risk. To estimate their risk, explicitly, compared with the other subgroups, we fitted an additional model, dividing the children into 4 groups (boys and girls, with and without high-grade reflux). We found that female patients with high-grade reflux had 55-fold greater odds of scarring compared with male patients without reflux (95% confidence interval: 7, 464) and 5-fold greater than male patients with high-grade reflux (95% confidence interval: 1, 30).
DISCUSSION
A recent review of the literature regarding prognostic indicators of renal deterioration in children with spinal dysraphism revealed multiple conflicting ideas and little in the way of clinical guidelines. Many studies focus on urodynamics, with and without video and/or electromyography, to characterize patients who will progress to renal deterioration (7–11). A lack of strict definitions for true renal deterioration in all studies has made comparison difficult. In our study, renal cortical loss, which is irreversible, was considered deterioration. On the other hand, hydronephrosis and reflux, which are reversible, were deemed potential risk factors for cortical loss.
In our study of 272 children with spinal dysraphism, we did not identify any urodynamic parameters that correlated with renal cortical loss. The San Diego group has the largest study evaluating urodynamics in children with spinal dysraphism (1). In this large study of 283 children, the definition of renal deterioration included hydronephrosis (either persistent or worsening), evidence of renal scarring or cortical thinning, or elevated serum creatinine for age. Even with this broad definition, they were unable to find any significant urodynamic parameters that were associated with renal deterioration. They did find an association between female sex and reflux, as did our study and others (2,12–14). Importantly, most children in that study were not started on CIC in the neonatal period.
Numerous studies have shown the association of urodynamic parameters with upper tract dilation and reflux (7–9,14). There have also been studies showing no correlation (1). Comparison of studies is impossible because of the multitude of urodynamic parameters used, including detrusor sphincter dyssynergia, compliance, detrusor leak point pressure, maximum urethral closing pressure, and detrusor instability. To further complicate the issue, few studies distinguish which patients are using CIC or antispasmodic agents.
The institution of CIC has been clearly shown to improve hydronephrosis and secondary reflux. Klose et al (15) examined 130 patients in their spina bifida clinic, 25 (19%) of which had either reflux or hydronephrosis without reflux. They reported resolution or improvement in 92% of patients started on CIC with or without anticholinergic medication. The resolution or improvement was stable at 41 months of follow-up. Similar results have been shown by Geraniotis et al (4) and Edelstein et al (16). Unfortunately, high-grade reflux seems to have minimal to no response to CIC (17,18).
The University of Michigan group found no association between grade of reflux and rate of spontaneous resolution (19). They further reported no correlation between grade of reflux at presentation and patient outcome defined by development of moderate to severe hydronephrosis, absence of renal growth, or a small kidney. This group also noted that both hydronephrosis and reflux are reversible, albeit only if leak point pressure is maintained at less than 40 cmH2 O.
The Washington DC group looked specifically at renal scarring with nuclear scintigraphy (13). Of this study population, 40% (72 of 180 children) had demonstrable reflux. Of the children with cortical loss, 75% had reflux. In our study, 90% of patients with cortical loss had reflux. Conversely, we found that 41% of children with high-grade reflux had evidence of cortical loss. On the other hand, less than 2% of children without reflux had cortical loss.
In our study, girls constituted two thirds of patients with high-grade reflux and 85% of children with cortical loss. Similar results have been reported by the Washington DC and San Diego groups (1,13). Our study showed that the risk (odds ratio) of renal scarring is independently associated with being a girl (6-fold) and having high-grade reflux (36-fold). Bivariate models suggest that the risks of high-grade reflux may be accounted for in part by its more frequent occurrence in girls, yet even within girls, reflux substantially increases the risk (18-fold). Girls with reflux have a 55-fold increased risk of cortical loss compared with boys without reflux.
We found that, although UDS parameters were associated with reflux and/or hydronephrosis, they have no association with cortical loss. Most surprisingly, hydronephrosis without reflux had absolutely no association with cortical loss. We theorize that these results are because of our near universal implementation of CIC in the neonatal period (3).
Some of our patients presented at a later age and thus had delayed initiation of CIC. Multivariate logistical regression analysis showed an association of renal cortical loss with the institution of CIC after 1 year of age (P = 0.06). We found that early institution of CIC has almost completely prevented the evolution of nonrefluxing hydronephrosis in infants. For the rare infant with persistent hydronephrosis and/or high-grade reflux despite CIC, vesicostomy has been beneficial (20).
In the past, UDS was used to determine which “high-risk” patients would need CIC (1,7,9). We support early intervention of CIC in all patients and agree with more recent studies that showed that CIC is well accepted, safe, and prevents the development of reflux and hydronephrosis (4,21–23). Despite CIC, some patients have persistent bladder hostility and require vesicostomy or augmentation. Neonatal CIC does not obviate the use of UDS, but rather, changes the indications and frequency of need.
CONCLUSION
In contrast to other studies, when we evaluated 272 children with spinal dysraphism, we used hydronephrosis and reflux, which are reversible, as risk factors rather than outcome measures. Renal cortical loss, which is irreversible, was the only measured outcome. After analyzing the data with descriptive, univariate, bivariate, and multivariate tools, a clear association of high-grade reflux, female sex, and delayed CIC with renal cortical loss was evident. Although hydronephrosis has often been synonymous with upper tract deterioration, we found hydronephrosis without reflux is not a risk factor for true renal deterioration. Although urodynamics do prognosticate hydronephrosis, no UDS parameters predicted cortical loss in our patients. The delayed initiation of CIC (after 1 year) was found to be a risk factor for renal deterioration in our bivariate analysis (with age and sex controlled) and approached statistical significance in our multivariate model. The early initiation of CIC is both intuitively appealing and supported by a majority of the current literature. We believe the clinical management and future studies of children with myelodysplasia should focus much more attention on high-grade reflux, female sex, and early initiation of CIC, with a stricter more universal definition of renal deterioration.
REFERENCES
- Teichman JM, Scherz HC, Kim KD, Cho DH, Packer MG, Kaplan GW. An alternative approach to myelodysplasia management: aggressive observation and prompt intervention. J Urol. 1994;152:807–811. doi: 10.1016/s0022-5347(17)32716-7. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Polse S. Renal deterioration in myelodysplastic children: urodynamic evaluation and clinical correlates. J Urol. 1998;159:1657–1661. doi: 10.1097/00005392-199805000-00084. [DOI] [PubMed] [Google Scholar]
- Bauer SB. Clean intermittent catheterization of infants with myelodysplasia. In: Kurzrock EA, editor. Dialogues in Pediatric Urology. Pearl River, NY: William J. Miller; 2000. pp. 1–8. [Google Scholar]
- Geraniotis E, Koff SA, Enrile B. The prophylactic use of clean intermittent catheterization in the treatment of infants and young children with myelomeningocele and neurogenic bladder dysfunction. J Urol. 1988;139:85–86. doi: 10.1016/s0022-5347(17)42300-7. [DOI] [PubMed] [Google Scholar]
- Kaplan WE, Firlit CR. Management of reflux in the myelodysplastic child. J Urol. 1983;129:1195–1197. doi: 10.1016/s0022-5347(17)52637-3. [DOI] [PubMed] [Google Scholar]
- Kasabian NG, Bauer SB, Dyro FM, Colodny AH, Mandell J, Retik AB. The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child. 1992;146:840–843. doi: 10.1001/archpedi.1992.02160190072024. [DOI] [PubMed] [Google Scholar]
- Bauer SB, Hallett M, Khoshbin S, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA. 1984;252:650–652. [PubMed] [Google Scholar]
- McLorie GA, Perez-Marero R, Csima A, Churchill BM. Determinants of hydronephrosis and renal injury in patients with myelomeningocele. J Urol. 1988;140:1289–1292. doi: 10.1016/s0022-5347(17)42027-1. [DOI] [PubMed] [Google Scholar]
- McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–209. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- Galloway NT, Mekras JA, Helms M, Webster GD. An objective score to predict upper tract deterioration in myelodysplasia. J Urol. 1991;145:535–537. doi: 10.1016/s0022-5347(17)38389-1. [DOI] [PubMed] [Google Scholar]
- Sidi AA, Dykstra DD, Gonzalez R. The value of urodynamic testing in the management of neonates with myelodysplasia: a prospective study. J Urol. 1986;135:90–93. doi: 10.1016/s0022-5347(17)45527-3. [DOI] [PubMed] [Google Scholar]
- Bauer SB, Colodny AH, Retik AB. The management of vesicoureteral reflux in children with myelodysplasia. J Urol. 1982;128:102–105. doi: 10.1016/s0022-5347(17)52774-3. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Rushton HG, Belman AB, Kass EJ, Majd M, Shaer C. Renal scarring and vesicoureteral reflux in children with myelodysplasia. J Urol. 1990;144:541–544. doi: 10.1016/s0022-5347(17)39517-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Shinno Y, Kakizaki H, Matsumura K, Koyanagi T. Relevance of detrusor hyperreflexia, vesical compliance and urethral pressure to the occurrence of vesicoureteral reflux in myelodysplastic patients. J Urol. 1992;147:413–415. doi: 10.1016/s0022-5347(17)37253-1. [DOI] [PubMed] [Google Scholar]
- Klose AG, Sackett CK, Mesrobian HG. Management of children with myelodysplasia: urological alternatives. J Urol. 1990;144:1446–1449. doi: 10.1016/s0022-5347(17)39763-x. [DOI] [PubMed] [Google Scholar]
- Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500–1504. [PubMed] [Google Scholar]
- Kass EJ, Koff SA, Diokno AC. Fate of vesicoureteral reflux in children with neuropathic bladders managed by intermittent catheterization. J Urol. 1981;125:63–64. doi: 10.1016/s0022-5347(17)54902-2. [DOI] [PubMed] [Google Scholar]
- Sidi AA, Peng W, Gonzalez R. Vesicoureteral reflux in children with myelodysplasia: natural history and results of treatment. J Urol. 1986;136:329–331. doi: 10.1016/s0022-5347(17)44856-7. [DOI] [PubMed] [Google Scholar]
- Flood HD, Ritchey ML, Bloom DA, Huang C, McGuire EJ. Outcome of reflux in children with myelodysplasia managed by bladder pressure monitoring. J Urol. 1994;152:1574–1577. doi: 10.1016/s0022-5347(17)32478-3. [DOI] [PubMed] [Google Scholar]
- Morrisroe SN, O'Connor RC, Nanigian DK, Kurzrock EA, Stone AR. Vesicostomy revisited: the best treatment for the hostile bladder in myelodysplastic children? BJU Int. 2005;96:397–400. doi: 10.1111/j.1464-410X.2005.05638.x. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Moore KN, Voaklander DC, Mix LW. Complications associated with clean intermittent catheterization in children with spina bifida. J Urol. 2004;171:2420–2422. doi: 10.1097/01.ju.0000125200.13430.8a. [DOI] [PubMed] [Google Scholar]
- Hopps CV, Kropp KA. Preservation of renal function in children with myelomeningocele managed with basic newborn evaluation and close followup. J Urol. 2003;169:305–308. doi: 10.1016/S0022-5347(05)64112-2. [DOI] [PubMed] [Google Scholar]
- Wu HY, LS Baskin LS, Kogan BA. Neurogenic bladder dysfunction due to myelomeningocele: neonatal versus childhood treatment. J Urol. 1997;157:2295–2297. [PubMed] [Google Scholar]