Abstract
Background/Objective:
To determine the body composition of adolescents with spinal cord injury (SCI) and to assess whether established cutoff values for obesity determined by body mass index (BMI) are valid for this population.
Methods:
Sixty patients, aged 10–21 years, with traumatic SCI (50 with paraplegia and 10 with tetraplegia) were compared with 60 gender-, age-, and BMI-matched controls (CTRL). Dual-energy x-ray absorptiometry was used to estimate regional and total bone mineral content, lean tissue mass, fat tissue mass, and body fat percentage. BMI was calculated from measured weight and stature (kg/m2).
Results:
Total percent body fat was significantly higher in the paraplegia group (31.4% ± 1.2%; mean ± SE) than in the tetraplegia and CTRL groups (25.7% ± 2.7% and 22.9% ± 1.1%, respectively). This change in percent total body fat was associated with a reduction of lean tissue mass in the paraplegia (37.6 ± 1.1 kg; mean ± SE) and tetraplegia (32.8 ± 2.5 kg) subjects as compared to the CTRL group (46.2 ± 1.0 kg; P < 0.001). Total fat mass was significantly greater in the paraplegia group (19.3 ± 1.3 kg) than the CTRL and tetraplegia groups (14.9 ± 1.2 kg and 11.7 ± 3.0 kg, respectively). Regional measurements revealed that the greatest reduction of lean tissue mass in the SCI subjects occurred in the lower extremities, followed by the trunk. As a result of these changes in body composition, the optimal BMI for classifying obesity (trunk fat percent >30 in males and >35 in females) in subjects with SCI was 19 kg/m2 as compared to 25 kg/m2 in able-bodied subjects.
Conclusions:
Patients aged 10 to 21 years with SCI have significantly decreased lean tissue mass and bone mineral content, and increased fat mass. As a result, traditional BMI cutoff criteria significantly underestimate obesity in this population. New clinically applicable criteria to define obesity should be established for SCI children and adolescents with SCI.
Keywords: Spinal cord injuries, Child, Adolescence, Paraplegia, Tetraplegia, Obesity, Body mass index, Body composition, Dual energy x-ray absorptiometry
INTRODUCTION
While obesity has reached epidemic proportions in the general US population, a growing number of research studies suggest even greater cause for concern among persons with disabilities (1–3). Weil et al (1) showed that adults with disabilities had a 66% higher rate of obesity compared with those without disabilities (24.9% vs 15.1%). Analysis of data from the 1998–1999 Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention found that people with disabilities, regardless of age, sex, race, or ethnicity, had higher rates of obesity than people without disabilities (2). Most recently, Rimmer and Wang (3) found that people with disabilities had significantly higher rates of overweight, obesity, and extreme obesity compared with people without disabilities. They reported that the frequency of extreme obesity was approximately 4 times higher among people with disabilities than the general population.
Obesity is recognized as a predictor of morbidity and mortality due to its role in chronic diseases including type II diabetes, cardiovascular disease, and stroke (4–6). Recently, it has been shown that many of the disorders associated with obesity occur prematurely and at a higher prevalence in the population with spinal cord injury (SCI) than in the able-bodied population. Adults with SCI have higher rates of carbohydrate intolerance, insulin resistance (7–10), lipid abnormalities (11–13), and heart disease (14) than the able-bodied population does, and these factors may contribute to the reduced lifespan of individuals with SCI (15).
Most of the aforementioned studies used body mass index (BMI, kg/m2) as a surrogate measure of obesity. BMI has the advantage of being a relatively simple tool, requiring only the measurement of height and weight. Although BMI is a reasonable measure of overall adiposity in the general population, there is reason to question similar application to all populations. Studies have called into question the applicability of conventional BMI cutoff values for a number of groups, including the Asian population (16), postmenopausal women (17), and persons with SCI (18). Several studies have shown that BMI is an insensitive marker of obesity in subjects with SCI, that it explains less of the variance in measured body fat than it does in the able-bodied population, and that it is inconsistently related to coronary heart disease (CHD) risk factors (18). Spungen et al found that persons with SCI at any BMI were fatter, demonstrating significantly less lean and more adipose tissue for any given age compared with controls (19). Jones et al found that men with SCI who did not appear obese had significantly greater adiposity than able-bodied men of similar age, height, and weight (20).
However, no studies have examined the body composition of children and adolescents with SCI. Accurate body composition measurements are necessary to evaluate whether BMI can be used as a surrogate measure of obesity in children and adolescents with SCI. Dual energy x-ray absorptiometry (DXA) has been shown to be a valid measure of regional and total body lean tissue, fat tissue, and bone mineral content and can be used as a reliable reference method (21–24).
The hypothesis of this study was that children and adolescents with paraplegia or tetraplegia due to SCI would have a significant difference in their body composition when measured by DXA as compared to able-bodied controls. Specifically, we hypothesized that children and adolescents with SCI would have significantly reduced lean tissue mass and significantly higher fat mass than gender-, age-, and BMI-matched controls. These alterations in body composition would lead the optimal obesity cutoff points of BMI in terms of sensitivity and specificity to be significantly higher in able-bodied control subjects than in age- and gender-matched children and adolescents with SCI.
METHODS
Subjects
Children and adolescents who were being followed at the SCI Clinics at the Shriners Hospital for Children Northern California and the University of California Davis Medical Center were invited to participate in a comprehensive study to assess body composition and fitness in subjects with paraplegia. To meet the inclusion criteria for the study all SCI subjects were required to be aged 10 to 21 years, be classified as thoracic to L4 and ASIA impairment classification A or B according to the standard American Spinal Injury Association (ASIA) classification (25), have no volitional control of the muscles below the sacral area, have full-time reliance on a wheelchair, and be >12 months from date of injury. The following exclusion criteria precluded subjects from being enrolled into the study: any surgical procedures within past 4 months, cognitive impairment that would interfere with testing procedures, and pregnancy. Able-bodied control subjects were recruited from the community through fliers, announcements, and word of mouth. The Human Subjects Review Committees at the Shriners Hospital for Children Northern California and the University of California Davis approved the study, and testing was conducted in compliance with the approved protocol. All subjects under 18 and their parents provided written assent or consent before participating in the study. Subjects 18 years or older provided written consent.
Fifty subjects (21 female and 29 male) with complete thoracic to L4 paraplegia due to SCI met the inclusion criteria for the study. Sixteen subjects had lesions between the T1 and T6 levels, 20 subjects had lesions between the T7 and L1 levels, 10 had lesions between the L1 and L2 levels, and 4 had lesions at the L3 or L4 levels. Ten adolescents (2 female and 8 male) with complete tetraplegia due to SCI at the C6 to C8 level were entered into the study. Sixty control subjects who were participating in a larger fitness study were matched for gender, age (±1 y), and BMI (±3 kg/m2) with each of the SCI subjects to ensure homogeneity between the groups. The subjects were instructed to avoid unusual physical activity for the 12 hours prior to testing and to fast for at least 8 hours prior to testing. Subjects were asked to void their bladders 1 hour before testing. Testing was initiated from 7 am to 9 am.
Anthropometrics
For all subjects who were able to stand erect, height was measured to the nearest centimeter with a wall-mounted stadiometer. For subjects unable to stand erect, stature was measured with a supine stadiometer. Every effort was made to account for any contractures. Weight of the subject and the wheelchair was obtained to the nearest kilogram with a wheelchair balance scale (Detecto, St Louis, MO) with the subject in the wheelchair. Then the wheelchair was weighed by itself and subtracted from the total weight. Able-bodied subjects were weighed standing on the same wheelchair balance scale. BMI was calculated from measured weight and height.
Body Composition by DXA
Body composition measurements including estimates of regional and total body bone mineral content (BMC, g) (26–29), lean and fat tissue mass (kg), and percent fat were obtained by DXA using a Hologic QDR 4500A fan beam densitometer, using version 11.2.1 software (Hologic, Bedford, MA). BMI was calculated from measured weight and stature (kg/m2). Obesity has been defined as accumulation of fat mass greater than 20% to 30% of body weight for boys and 30% to 35% of body weight for girls (26–29). To determine whether subjects from this population were obese, percent trunk fat was determined using DXA, as it has been shown to be an excellent measure of central obesity and correlates very well with visceral fat area as measured by either computed tomography or magnetic resonance imaging (30). In this study, obesity was defined as a percent trunk fat by DXA of ≥30% in males and ≥35% in females, which corresponds to the 85th percentile in the population (31).
Research Design and Statistics
A cross-sectional comparative study design was employed. An analysis of covariance adjusted for age and gender was used to assess overall differences among the paraplegic, tetraplegic, and control groups. The Tukey HSD multiple comparison analysis was used to examine differences between the groups. Regression analyses were used to determine the association between body mass index, percent total fat, and percent trunk fat in each of the groups. We examined the sensitivity and specificity of BMI as a measure of central obesity as measured by DXA-determined percent trunk fat. Receiver operating characteristic (ROC) curves were used to identify the optimum tradeoff between sensitivity and specificity for cutoff points in the BMI distribution. The optimum cutoff point is considered to be the point where the ROC sharply turns. All statistical analyses were performed using Systat 11.0 software, and significance was accepted at P < 0.05.
RESULTS
As shown in Table 1, there were no significant differences in the height, weight, BMI, or BMI-percentile adjusted for age (32) between the paraplegic group and its gender-, age-, and BMI-matched controls. There were also no significant differences in age, height, and weight between the tetraplegia group and the CTRL group. However, the tetraplegia group had a significantly lower BMI and significantly lower BMI-percentile adjusted for age than the CTRL group. This occurred because 6 of the 10 subjects with tetraplegia had a BMI that was less than the second percentile when adjusted for age and gender.
Table 1.
Physical Characteristics of Children and Adolescents With Spinal Cord Injury and Controls*
The regional and total body composition measures obtained by DXA are shown in Table 2. The subjects in the paraplegia group had 38% less lean tissue mass in the legs (P < 0.0001) and 11% less lean tissue mass in the trunk (P < 0.003) than the CTRL group did. These losses resulted in an 18% reduction in total body lean tissue mass (P < 0.0001). The lean tissue mass of every region—arms, legs, trunk, and total body—was significantly less in the tetraplegia group than the CTRL group. The tetraplegia group had 35% less lean tissue mass in the arms (P < 0.0001), 37% less lean tissue mass in the legs (P < 0.0001), and 26% less lean tissue mass in the trunk (P < 0.0001), resulting in a 29% loss of lean tissue mass in the total body (P < 0.0001). The tetraplegia group had 36% less lean tissue mass in the arms (P < 0.0001) and 16% less lean tissue mass in the trunk (P < 0.05) than the paraplegia group did.
Table 2.
Regional and Total Body Composition Results*
The BMC of the paraplegia group was 40% lower in the legs (P < 0.0001), while there were no significant differences in the arms or trunk as compared to the CTRL group. As a result, the total BMC of the paraplegia group was 16% lower than that of the CTRL group (P < 0.0001). The tetraplegia group had significantly less BMC in the arms (−25%; P < 0.0001), legs (−46%; P < 0.0001), and trunk (−30%; P < 0.0001) as compared to the CTRL group. The tetraplegia group had significantly less BMC in the arms (−33%; P < 0.002) and trunk (−30%; P < 0.004) as compared to the paraplegia group.
There were no differences between the slopes of the regression between lean tissue mass and BMC in the paraplegia group and the tetraplegia group, when examined as the total lean tissue or by each region. As shown in Figure 1, the total lean tissue was highly correlated to the total bone mineral content in the SCI group (paraplegia and tetraplegia groups combined) and CTRL group. However, the slope of the regression between lean tissue mass and BMC was significantly higher in the CTRL group as compared to the SCI group (Figure 1). This increased slope was also significant for each of the regions. The arm lean tissue mass was positively related to arm BMC in the SCI group (r = 0.84) and CTRL group (r = 0.89). The leg lean tissue mass was positively related to total BMC in the SCI group (r =0.74) and CTRL group (r = 0.88). The trunk lean tissue mass was positively related to trunk BMC in the SCI group (r = 0.71) and CTRL group (r = 0.87).
Figure 1. Relationship between total lean tissue and total bone mineral content for the SCI group (paraplegia and tetraplegia groups combined: Total Lean, kg = 0.0136 ×Total BMC, g + 11.2 g, dashed line; r = 0.81, P < 0.0001) and control group (Total Lean, kg =0.0187 ×Total BMC, g +3.3 g, solid line; r =0.89, P < 0.0001).
The paraplegia group had 28% more fat mass in the legs (P < 0.02), 35% more fat mass in the trunk (P < 0.04), and no significant difference in fat mass in the arms as compared to the CTRL group. Although the tetraplegia group had lower fat mass in the arms, legs, trunk, and total body than the CTRL group, the variance in the tetraplegia group was so large that these differences were not significant. The paraplegia group had 38% more fat in the legs and 39% more total body fat than the tetraplegia group did.
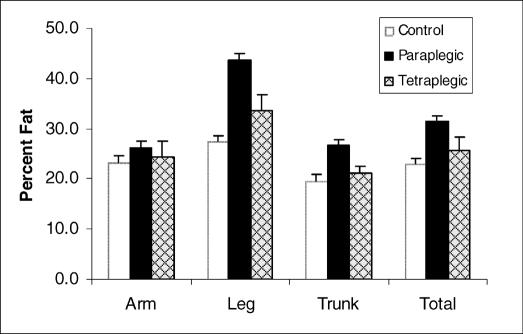
As a result of the loss in lean muscle tissue in the leg and trunk and the increase in total fat mass in the leg and trunk, the paraplegia group had a 36% higher trunk fat percentage (P < 0.001), a 60% higher leg fat percentage (P < 0.0001), and a 37% higher total body fat percentage (P < 0.0001) as compared the CTRL group (Figure 2). No differences in percent fat were observed in the arms. Although the arm fat percentage, trunk fat percentage, and total body fat percentage were higher in the tetraplegia group than the CTRL group, the variances were so large that these differences were not significant. Despite the large variance, the tetraplegia group had a significantly lower leg fat percentage than did the paraplegia group.
Figure 2. Regional and total body percent fat mass in the CTRL, paraplegia, and tetraplegia groups. Values are means ± SEM.
The changes in body composition observed in the subjects with SCI had profound effects on the relationship between BMI and total fat percentage as compared to the CTRL subjects, although there were no differences between the paraplegia and tetraplegia groups. Results from the analysis of covariance revealed that the percent total fat observed in the SCI group was significantly higher at any BMI as compared to the control group (P < 0.0001). This analysis resulted in an average of 38% ± 4% more fat tissue per unit BMI in the SCI group compared with the control group (Figure 3).
Figure 3. Relationship between body mass index and total percent fat in the control, paraplegia, and tetraplegia groups. There were no differences between the paraplegia and tetraplegia groups. However, subjects with SCI had, on average, 38 ± 4% more fat tissue per unit BMI than the control group did.
To determine the optimal cutoff points for BMI corresponding to the criterion value (percent trunk fat by DXA), a receiver operator characteristic (ROC) curve was generated (Figure 4). The BMI that maximized the sensitivity and specificity corresponding to percent trunk fat of ≥30% in male subjects and ≥35% in female subjects was 25 kg/m2 in the control group and 19 kg/m2 in the SCI group. Table 3 shows the sensitivity (true-positive rate) and specificity (true-negative rate) used to create the ROC analysis. The area under the curve was 0.962 for the control group and 0.887 for the SCI group, which shows that BMI is a good predictor of obesity in both groups as long as optimal cutoff points are used.
Figure 4. Receiver operator characteristic curve for prediction of central obesity for BMI in control subjects (♦) and spinal cord injured subjects (□). The optimal cutoff value for BMI was 25 kg/m2 in the control group and 19 kg/m2 in the group with SCI. The area under the curve was 0.962 for the control group and 0.887 for the SCI group.
Table 3.
Receiver Operator Curve Analysis for Prediction of Central Obesity*
DISCUSSION
Childhood obesity has increased at alarming rates in the last decade. The recent identification of major abnormalities of carbohydrate metabolism in obese children and adolescents underscores the importance of childhood obesity as a major health priority. This is especially true in individuals with SCI.
One of our primary hypotheses was that children and adolescents with SCI develop significant alterations in body composition when compared to age-, gender-, and BMI-matched controls that result in significant obesity. Results from this study clearly show that the major cause of the increased obesity observed in the SCI subjects was the reduction of lean muscle tissue and bone mineral content and a concomitant increase in fat tissue. These data are in agreement with previous findings in adults with SCI that suggest that muscle is replaced by fat tissue, especially in the legs (19,33,34). Regionally, the most significant loss of lean tissue occurred in the legs and trunk. However, the loss of lean tissue is likely to be even greater because DXA measurements have been shown to underestimate the loss of lean muscle tissue when compared to more sensitive measurements, such as magnetic resonance imaging and quantitative computed tomography (30).
The absolute lean tissue mass of the trunk and arms was significantly lower in the tetraplegia group as compared to the paraplegia group. A potential reason why the paraplegia group was not significantly different from the control group, while significant differences were observed in the tetraplegia group, is because the subjects with paraplegia are likely to use their arms for activities of daily living, such as pushing a wheelchair, while subjects from the tetraplegia group would not place these exercise demands on their arms. Unfortunately, the tetraplegia group was significantly lighter than their BMI-matched controls. This occurred because a majority of the subjects with tetraplegia had a BMI adjusted for age that was less than the second percentile and we were unable to recruit controls whose BMI was that low. The restrictions in arm movement may have limited the caloric intake of the tetraplegic subjects and may account for their reduced weight and extremely low BMI values, low lean tissue mass, and low fat mass.
SCI resulted in a significant reduction in the total bone mineral content in every region in both paraplegia and tetraplegia subjects as compared to the controls. This loss of bone mineral content has been associated with increased risk of fractures (35). In contrast to results reported by Spungen et al (19), very strong associations were found between bone mineral content and lean muscle tissue, both regionally and overall in both the SCI and control subjects. Since our subjects were young, they might not have experienced the disparate loss of lean tissue that occurs with many years' duration of injury.
In 2003, the American Academy of Pediatrics published guidelines recommending that clinicians screen patients for obesity based on BMI and offer appropriate behavioral interventions and counseling to promote sustained weight loss for those who are obese (36,37). It is essential to have appropriate screening tools to assess obesity in persons with disability. As a result of the significant alterations in body composition, the standard classification of BMI is not accurate in children and adolescents with SCI. In our sample, 26.7% of the subjects from the paraplegia group were obese (percent trunk fat >35% for females and >30% for males), whereas only 10% of the controls were obese, even though they had the similar weights and BMI values. In our sample, the receiver operator curve (ROC) showed that the most accurate cutoff value for obesity was a BMI of 19 kg/m2 in children and adolescents with SCI and 25 kg/m2 for able-bodied children and adolescents. The ROC curves also revealed that BMI is a more sensitive and accurate predictor of obesity in the control group than in the SCI group. These data show that BMI, especially when the standard cutoff point of ≥30 kg/m2 is used to classify obesity, significantly underestimates the frequency of obesity in children and adolescents with SCI.
The identification of obesity among children and adolescents with SCI is of critical importance for prevention of long-term obesity-related morbidity in this population. Dopler-Nelson et al (38) recently reported that more than 90% of obese SCI subjects from a regional SCI clinic met the criteria for metabolic syndrome, which puts them at risk for type II diabetes, cardiovascular disease, and stroke. Identification of obese patients at risk for metabolic syndrome using easily obtainable clinical criteria for obesity should be a high priority for clinical care.
Although DXA has become the primary accepted research tool for the evaluation of body composition in children, it has several limitations that reduce its validity in measuring the body composition of children with SCI. Densitometry may not be entirely accurate in children with SCI because of changes in body composition that accompany their growth, maturation, and recovery from injury. Estimating the fat and lean tissue composition in bone-containing pixels is complex and requires assumptions because the amount of fat and lean tissue is not uniform throughout the body. There is more adipose tissue in the subcutaneous regions at the surface and edges of the body and there is more lean tissue, particularly skeletal muscle, immediately adjacent to the appendicular bones. Therefore, manufacturers of DXA systems have developed software algorithms, such as the weighted linear distribution model, to estimate the amount of fat and lean tissue in pixels containing bone (39). However, children with SCI may not fit the assumptions that have been used to develop these models because they have significant muscle atrophy and corresponding loss of bone and girth of their lower extremities. Clasey and Gater (40) have recently questioned the validity of using DXA in persons with SCI because more than 60% of the 21,000 pixels in a typical whole body scan contain bone. Therefore, a significant number of the values used to determine the amount of fat and lean tissue in SCI subjects are based upon assumptions that have not been validated in this population. Thus, we cannot be totally assured that the body composition values that we have reported are absolutely valid in this population. Further research with a 4-compartment model and larger sample sizes should be performed to develop standard cutoff criteria for obesity in children with SCI. Despite these limitations, our data clearly show very significant differences in the regional body composition of children with SCI as compared with able-bodied controls and show how these changes in body composition alter the interpretation of BMI in these subjects.
CONCLUSION
This study showed that patients aged 10 to 21 years with SCI have significantly reduced lean tissue mass and significantly higher fat mass than gender-, age-, and BMI-matched controls. Due to these alterations in body composition, BMI significantly underestimates the level of obesity in this population, and the standard cutoff criterion for obesity of 30 kg/m2 should not be used. Since obesity is very common in this population and can lead to significant morbidity, more accurate methods of screening should be employed for all subjects who may be at risk. Due to limitations of measuring body composition by DXA in children with SCI, further studies should be conducted using a 4-compartment model to determine appropriate measures of obesity in this population.
Footnotes
Supported in part by Shriners Hospitals for Children Project 8600: Exercise and Dietary Intervention in Obese Children with Paraparesis Due to Spinal Cord Dysfunction; National Institute of Disability and Rehabilitation Research Grant #H133B031118: Rehabilitation Research and Training Center in Neuromuscular Diseases: Enhancing Health, Function, and Quality of Life; National Institute of Child Health and Human Development Grant # RO1 HD35714: Child Mobility: Role of Strength, Body Fat, and Energy Cost.
REFERENCES
- Weil E, Wachterman M, McCarthy EP, et al. Obesity among adults with disabling conditions. JAMA. 2003;288:1265–1268. doi: 10.1001/jama.288.10.1265. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention State-specific prevalence of obesity among adults with disabilities—eight states and the District of Columbia, 1998–1999. MMWR. 2002;51:805–808. [PubMed] [Google Scholar]
- Rimmer JH, Wang E. Obesity prevalence among a group of Chicago residents with disabilities. Arch Phys Med Rehabil. 2005;86:1461–1464. doi: 10.1016/j.apmr.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Vanhala M, Vanhala P, Kumpusalo E, Halonen P, Takala J. Relation between obesity from childhood to adulthood and the metabolic syndrome: population based study. BMJ. 1998;317(7154):319–320. doi: 10.1136/bmj.317.7154.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Sundstrom J, Vallhagen E, Riserus U, et al. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29:1673–1674. doi: 10.2337/dc06-0664. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Raza M, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med. 1992;59:163–168. [PubMed] [Google Scholar]
- Duckworth WC, Jallepalli P, Solomon SS. Glucose intolerance in spinal cord injury. Arch Phys Med Rehabil. 1983;64:107–110. [PubMed] [Google Scholar]
- Karlsson AK. Insulin resistance and sympathetic function in high spinal cord injury. Spinal Cord. 1999;37:494–500. doi: 10.1038/sj.sc.3100844. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30:697–703. doi: 10.1038/sc.1992.136. [DOI] [PubMed] [Google Scholar]
- Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil. 1986;67:445–450. [PubMed] [Google Scholar]
- LaPorte RE, Brenes G, Dearwater S, et al. HDL cholesterol across a spectrum of physical activity from quadriplegia to marathon running. Lancet. 1983;1:1212–1213. doi: 10.1016/s0140-6736(83)92482-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Raza M, Spungen AM, Machac J. Cardiac stress testing with thallium-201 imaging reveals silent ischemia in individuals with paraplegia. Arch Phys Med Rehabil. 1994;75:946–950. [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center Spinal Cord Injury Facts and Figures at a Glance—June 2006. Spinal Cord Injury Information Network 2006; Available at http://www.spinalcord.uab.edu. Accessed May 2, 2007.
- Goh VH, Tain CF, Tong TY, Mok HP, Wong MT. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J Lipid Res. 2004;45:1892–1898. doi: 10.1194/jlr.M400159-JLR200. [DOI] [PubMed] [Google Scholar]
- Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes (Lond) 2006;30:837–843. doi: 10.1038/sj.ijo.0803208. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84:1068–1071. doi: 10.1016/s0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Chan GM. Performance of dual-energy x-ray absorptiometry in evaluating bone, lean body mass, and fat in pediatric subjects. J Bone Miner Res. 1992;7:369–374. doi: 10.1002/jbmr.5650070403. [DOI] [PubMed] [Google Scholar]
- Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of body-composition techniques against dual-energy x-ray absorptiometry in young children. Am J Clin Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- Gutin B, Litaker M, Islam S, Manos T, Smith C, Treiber F. Body-composition measurement in 9–11-y-old children by dual-energy x-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am J Clin Nutr. 1996;63:287–292. doi: 10.1093/ajcn/63.3.287. [DOI] [PubMed] [Google Scholar]
- Rico H, Revilla M, Villa LF, Hernandez ER, Alvarez dB, Villa M. Body composition in children and Tanner's stages: a study with dual-energy x-ray absorptiometry. Metabolism. 1993;42:967–970. doi: 10.1016/0026-0495(93)90008-c. [DOI] [PubMed] [Google Scholar]
- Ditunno JF. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 1992. [Google Scholar]
- Bray GA. In defense of a body mass index of 25 as the cutoff point for defining overweight. Obes Res. 1998;6:461–462. [PubMed] [Google Scholar]
- Lohman T, Going S. Assessment of body composition and energy balance. In: Lamb D, Murray R, editors. Perspective in Exercise Science and Sports Medicine. Carmel, IN: Cooper Publishing; 1998. pp. 61–105. [Google Scholar]
- Dwyer T, Blizzard CL. Defining obesity in children by biological endpoint rather than population distribution. Int J Obes Relat Metab Disord. 1996;20:472–480. [PubMed] [Google Scholar]
- Higgins PB, Gower BA, Hunter GR, Goran MI. Defining health-related obesity in prepubertal children. Obes Res. 2001;9:233–240. doi: 10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy x-ray absorptiometry and magnetic resonance imaging. J Appl Physiol. 2004;96:561–565. doi: 10.1152/japplphysiol.00207.2003. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Moreno LA, Blay MG, et al. Body composition in adolescents: measurements and metabolic aspects. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S54–S58. doi: 10.1038/sj.ijo.0802805. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88:1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- Maggioni M, Bertoli S, Margonato V, Merati G, Veicsteinas A, Testolin G. Body composition assessment in spinal cord injury subjects. Acta Diabetol. 2003;40(Suppl 1):S183–S186. doi: 10.1007/s00592-003-0061-7. [DOI] [PubMed] [Google Scholar]
- Garland DE, Maric Z, Adkins RH, Stewart CA. Bone mineral density about the knee in spinal cord injured patients with pathologic fractures. Contemp Orthop. 1993;26:375–379. [Google Scholar]
- US Preventive Services Task Force Screening for Obesity in Adults: Recommendations and Rationale. Agency for Healthcare Research and Quality. 2006. Available at: http://www.ahrq.gov/clinic/uspstf/uspsobes.htm. Accessed May 2, 2007.
- American Academy of Pediatrics Policy Statement. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- Dopler-Nelson M, Widman LM, Abresch RT, Stanhope K, Havel PJ, McDonald CM. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30:S130–S142. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord RH, Payne RK. Body composition by dual-energy X-ray absorptiometry—a review of the technology. Asian Pac J Clin Nutr. 1995;4:173–175. [PubMed] [Google Scholar]
- Clasey JL, Gater DR. Body composition assessment in adults with spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12:8–19. [Google Scholar]