Abstract
Background/Objective:
Spinal cord tumors are a relatively rare diagnosis, accounting for 1% to 10% of all pediatric central nervous system tumors. Understanding the etiology and clinical outcomes of these tumors is therefore very important. This study presents detailed information regarding clinical presentation, histological findings, outcomes, functional assessment, and management of a series of patients with this diagnosis.
Method:
Retrospective, descriptive study.
Subjects:
Thirty-five children with a final diagnosis of spinal cord tumor or mass, excluding dysraphism.
Results:
Neurodevelopmental tumors (dermoid tumors, epidermoid tumors, and teratomas) were the most common tumor type (31%), followed by astrocytomas (29%) and neuroblastomas (14%). Other types included schwannomas, meningiomas, giant cell tumors, extradural cystic masses, leukemic-related masses, and masses related to neurofibromatosis. Mean age at diagnosis was 6.6 years (SD = 5.5 y) and did not vary significantly by tumor type except for children with neuroblastoma (mean = 0.4 y, SD = 0.5 y). More boys (57%) were identified in the series than girls (43%); however, there was no association between tumor type and sex. Presenting complaints of pain were noted in 57% and were localized to the back, neck, or extremities. Extremity weakness was reported as an initial presenting symptom in 46%. Three children had scoliosis as a presenting issue and 14 had gait abnormalities. Regardless of treatment modality, mobility was retained in 83% of children with or without gait aids. Neurogenic bowel and/or bladder were present in 23% of the population.
Conclusions:
This study corroborates other studies indicating that intramedullary tumors are the predominant form of pediatric spinal cord tumor. This population, however, presented with an unusually large number of developmental tumors, contrary to several published studies. The disparity may be the result of this institution acting as a regional referral center, thus increasing the number of this type of patient. The population is too small to make any other conjecture. The predominance of astrocytomas and neuroblastomas among those patients with poor outcomes and the prevalence of developmental tumors suggest the need for broader investigation. Although, in general, spinal cord tumors are relatively rare, this preliminary study supports the need to further evaluate associations between tumor type, presenting symptoms, treatment, and functional outcome in children with spinal cord tumors.
Keywords: Myelopathy, neoplastic, Spinal cord tumor, extramedullary, developmental, extradural, Child, Outcome
INTRODUCTION
Tumors of the central nervous system are common in the pediatric population and constitute the second most prevalent tumor type of childhood. Within this group, spinal cord tumors are a relatively rare diagnosis and account for 1% to 10% of all pediatric central nervous system tumors (1–3). The most common spinal cord tumors are intramedullary (4). The gross classification of tumors based on anatomic location can be divided into 3 discrete areas. First are extradural tumors and masses, which localize to the area between the bony structures and the dura. Next are intradural masses and tumors, which are subdivided into extramedullary and intramedullary. Extramedullary refers to the area within the dura but not part of the spinal cord and intramedullary is within the spinal cord parenchyma. Different types of tumors and masses are predominantly found within these anatomic areas.
Extradural tumors can arise from bony elements, the meninges, or soft tissues. Neuroblastomas and sarcomas are frequently encountered along with bone tumors (5). Intradural extramedullary tumors can be meningial in origin or from distant sites and include meningiomas and schwannomas. Of this group most tend to be benign. Intradural intramedullary tumors can be derived from neuroepithelial tissues. These general groups are neuronal, glial, and primitive neuroepithelial. Neuronal tumors are generally gangliocytomas. Tumors from primitive neuroectoderm are referred to as PNETs and frequently are labeled medulloblastomas. Glial tumors are derived from supportive structures and include astrocytomas, ependymomas, and oligodendrogliomas (6).
Tumors and masses are also classified based on the histopathology of samples obtained through surgical resection or tissue biopsy. The predominance of these tumor types varies between adults and children. In children with intramedullary tumors, astrocytomas represent around 60% of tumors, ependymomas 30%, developmental tumors 4%, and then a group of other less frequently identified types (3,7). As noted by Kumar et al, his trend may be different in other countries, where developmental tumors seem to have a higher ratio (8).
Types of Tumors
Astrocytomas are categorized into 4 different classifications based on World Health Organization guidelines. Grade 1 is a pilocytic astrocytoma, grade 2 is a low-grade astrocytoma, grade 3 is an anaplastic astrocytoma, and grade 4 is a glioblastoma multiforme. Low-grade tumors tend to be the predominant type (7,9). They are most common in the thoracic region of the spinal cord (10). Ependymomas develop in the central canal of the spinal cord, particularly in the cervical region. There is an association of these types of tumors with neurofibromatosis type 2. There are 4 types of ependymomas but only 3 grades. Grade 1 is slow growing and histologically is either a myxopapillary ependymoma or a subependymoma (10). Grade 3 is anaplastic and grade 2 is in between.
Developmental tumors are a slow-growing group and include dermoid tumors, epidermoid tumors, and teratomas. They are generally benign, but can reoccur if resection is subtotal. The distinction between types is somewhat blurry. In general an epidermoid tumor is ectodermal in origin and contains a inner layer of stratified epithelium and a fibrous capsule (11). The dermoid tumor is an orderly group of ectodermal and mesodermal elements. It also has an epithelial lining and capsule but is more developed and has structures including hair, follicles, and sweat glands. Teratomas have more than one cell line present and tend to be less well defined and devoid of organization.
Intraspinal lipomas account for 1% of all spinal cord tumors, and up to 99% of them are associated with spinal dysraphism (12). Intraspinal lipomas can be divided into 3 main types: lipomyelomeningoceles (or lipoma of the conus medullaris), intradural lipomas, and fibrolipomas of the filum terminale. Intradural lipomas represent approximately 4% of all spinal lipomas (13). They can be either intramedullary or a combination of intramedullary and extramedullary (13). Most intradural lipomas occur in the cervical and thoracic spine. Several authors have found the cervical region to be most commonly involved in children (13). They are usually located in the dorsal part of the cord. They tend to result in neurologic deficits when they become large enough to exert mass effect. They most commonly present in the second and third decades of life, but 25% present within the first 5 years of life (14).
Neuroblastoma is a primitive tumor type with its origin from neural crest cells, which differentiate into sympathetic neurons. The primary site of these tumors is along the migration pathway, and the adrenal gland and sympathetic chain are most often involved (15). These tumors are typically extradural and can cause compression of the spinal cord, which may result in neurologic damage.
Clinical Presentation
Spinal cord tumors and masses can present with a variety of clinical signs and symptoms. One study on children younger than 3 years of age diagnosed with intramedullary tumors indicated that they presented with pain followed by motor regression, gait disturbance, torticollis, and kyphoscoliosis (16). In children with intradural extramedullary tumors, the most common reason for diagnostic workup was pain followed by limb weakness, sphincter dysfunction, and finally, sensory symptoms (8). The adult literature suggests the most common site for intramedullary lesions is the cervical area followed by thoracic and then conus (17–19).
Treatment of spinal cord tumors and masses is based on tumor type, but surgical resection is the mainstay. Factors identified as predictors of outcome with intramedullary tumors include the histologic grading of the tumor and the neurologic status at the time of surgery (20). Functional outcomes do not appear to be affected by the advent of a less than total resection (17).
METHODS
This retrospective review was conducted at a large regional tertiary care pediatric hospital that serves as a referral center for children with central nervous system tumors. With human subject oversight committee approval, records were reviewed for children with a primary diagnosis indicating a spinal cord tumor or mass within the preceding 6 years (2000–2006). Pertinent information relating to the patient's status at diagnosis, subsequent care, and outcome were noted (see Results). Functional changes were assessed retrospectively by the authors using the modified McCormick scale (8). The McCormick scale, a method of assessing tumor impact on function incorporating both motor and sensory components, is a tool frequently found in the neurosurgical literature for evaluating patients with intramedullary tumors/masses (21,22). This functional outcome tool has been modified for use in evaluating children with tumors (8). Summary data were compiled and analyzed using GraphPad InStat version 3.06 (32-bit) for Windows (GraphPad Software, San Diego, CA).
RESULTS
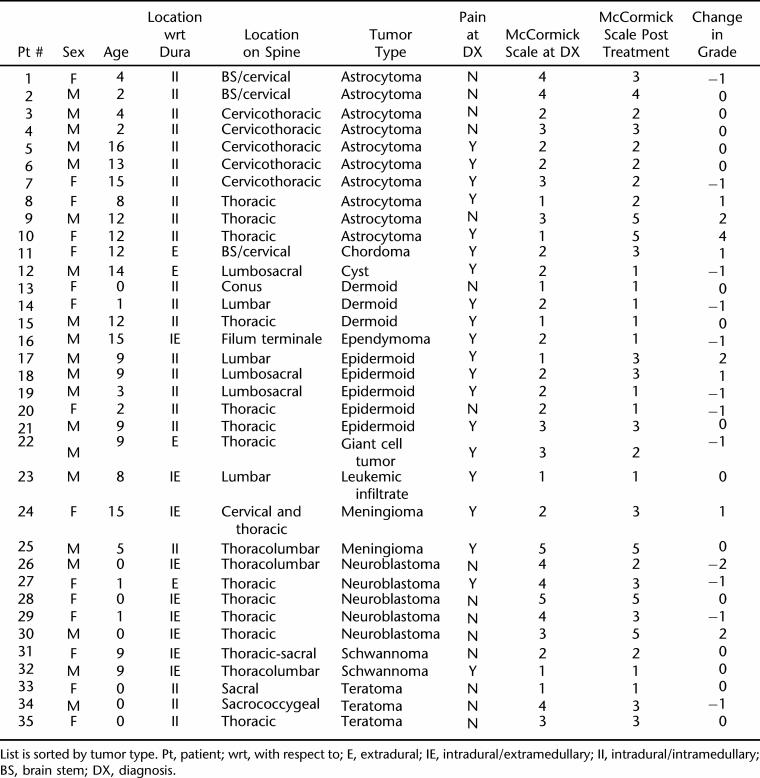
This is a retrospective review of patients who presented with spinal cord tumors and masses from 2000 through 2006 (Table 1). Tumors that were associated with dysraphism of the spinal cord were excluded. A total of 35 children with complete information were identified, and of this population, 15/35 (43%) were girls and 20/35 (57%) were boys. The mean age for all tumor types was 6.6 ± 5.5 (mean ± standard deviation) years.
Table 1.
Listing of Patients Identified for Study*
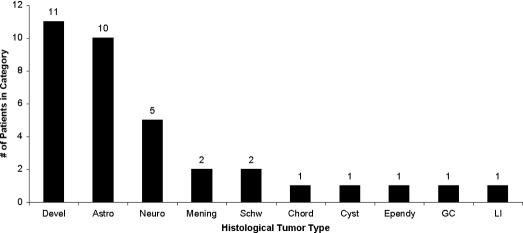
Ten different tumor types or masses were identified in this group. Neurodevelopmental tumors were the most common at 11/35 (31%), followed by astrocytomas 10/35 (29%), neuroblastomas 5/35 (14%), meningiomas 2/35 (6%), schwannomas 2/35 (6%), and chordoma, ependymoma, giant cell, leukemic infiltatrate, and cystic lesions each found in 1 patient (Figure 1). There were no lipomas in this population that were not associated with spinal dysraphism.
Figure 1. Distribution of patients by histologically defined tumor class. Devel, developmental tumors; Astro, astrocytomas; Neuro, neuroblastoma; Mening, meningioma; Schw, schwannoma; Chord, chordoma; Cyst, cyst; Ependy, ependymoma; GC, giant cell tumor; LI, leukemic infiltrate.
The anatomical classification of the tumors with respect to the dura was as follows: extradural in 8/35 (23%), intradural extramedullary 5/35 (14%), and intradural intramedullary 22/35 (63%). Tumors/masses identified in this study were most frequently localized along the neuraxis at the thoracic level (n = 21) followed by lumbar localization (n = 11). The remaining frequencies of localization were cervical (n = 8), sacral (n = 6), coccxygeal (n = 3), and brainstem (n = 2). Note that the majority of tumors spanned more than one spinal segment; therefore, the above represents how prevalent tumor is in a given segment and not a total on a per tumor basis. The 3 largest histologically grouped tumors (developmental, astrocytoma, and neuroblastoma) tended to group themselves along the neuraxis, with the astrocytomas being primarily cervicothoracic (n = 10, 2 with brainstem extension), neuroblastomas localizing to the thoracic spinal region (n = 5), and developmental tumors (dermoid, epidermoid, and teratoma) being identified primarily in the lumbosacral region (n = 11, 3 with thoracic extension/localization).
The presenting symptom of pain was present in 57% (20/35) of the population, weakness was noted in 16/35 (46%), and gait disturbance in 14/35 (40%). Scoliosis as the presenting etiology was only seen in 3/35 (9%). When pain was evaluated at diagnosis, there was no significant difference in the location of the tumor being either intramedullary vs extramedullary. There was no preponderance of pain with any particular type of tumor. The neuroblastoma group was rated as having no pain but this may be a reflection of age (mean age 0.4 ± 0.5 y), making it difficult to assess the presence of pain. Of this study group, 31/35 (89%) had some type of surgical decompression or resection.
Functional outcomes were evaluated, and 100% of children were able to participate in some or all activities of daily living (ADLs). Neurogenic bowel and bladder were present after treatment in 23% (8/32) of the population, and were most commonly associated with those children who had neuroblastomas followed by astrocytomas and teratomas. The majority (83%, 29/35) were able to ambulate with or without gait aids. Using a McCormick functional scale at the time of presentation, 8/35 (23%) were grade 1, 12/35 (34%) were grade 2, 7/35 (20%) were grade 3, 6/35 (17%) were grade 4, and 2/35 (6%) were grade 5. After treatment, the distribution based on McCormick functional grade changed as follows: 10/35 (29%) grade 1, 8/35 (23%) grade 2, 11/35 (31%) grade 3, 1/35 (3%) grade 4, and 5/35 (14%) grade 5. In terms of changes in functional status, 12 improved their scores, 8 declined in scores, and 15 had no changes. Of the children with a grade 5 outcome, the majority were neuroblastomas and astrocytomas. None of the children with grade 5 class had any functional changes.
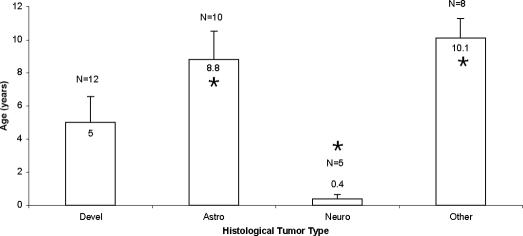
Histological tumor type distribution by age showed that those children less than two years of age all had neuroblastomas, teratomas, or dermoid tumors. The 2- to 5-year age group presented predominantly with astrocytomas and epidermoid tumors. The 6- to 10-year-old group had a mix of tumor types, and for those children >10 years of age, astrocytomas were the predominant subtype. There were significant differences in mean age associated with tumor type (P = 0.003); neuroblastoma patients were demonstrated to be younger than their counterparts with astrocytomas (Figure 2). There were no differences in mean age based on location of the tumor with respect to the dura (ie, extradural vs intradural), nor whether or not the tumor localized to the parenchyma of the spinal cord.
Figure 2. Mean age for each histologically defined tumor class. Classes were evaluated as a group if they contained 5 or more. The remaining were grouped into the “other” category. *, demonstrated significant difference; Devel, developmental tumors (dermoid, epidermoid, teratoma); Astro, astrocytoma; Neuro, neuroblastoma.
DISCUSSION
Spinal cord tumors and masses are a rare diagnosis in the pediatric population. The types of tumors seen in children tend to be different then the adult types. In evaluating the trends seen in this population, we had a large percentage of developmental tumors (dermoid, epidermoid, and teratomas) which differs from other, similar reports on tumors in this population (3,4,23,24). This does, however, counter a report by Townsend et al in a small case series of 10 patients (9). In addition, the majority of tumors identified in this study were intramedullary, which is in general agreement with previous published data (3,9,10,17,25,26). Specifically, astrocytomas in this study were abundant and restricted to the cervicothoracic region, and were intramedullary, which is in agreement with a report by Aguste and Gupta (10). Developmental tumors are predominantly found in the lumbosacral area, with the exception of a few in the thoracic area. Neuroblastoma tumors (neural crest derived) were all located in the thoracic region, consistent with documented cellular migration patterns.
All children diagnosed with spinal tumors had some type of presenting symptomatology, with pain being the most common. Pain was most frequently seen in the back, but a few patients presented with pain in the extremities. Pain was not associated with any particular tumor type; however, pain was noticeably absent in patients with neuroblastoma. This is not unexpected as the children typically present at such an early age where pain can be difficult to discern and localize. It is important to recognize that complaints of pain may be vague and nonspecific in infants and young children, which can result in a delay in diagnosis. Robertson reported on 2 children with intramedullary spinal cord tumors whose diagnoses were delayed for months due to atypical pain. One child presented with recurrent abdominal pain and the other had abrupt, paroxysmal but infrequent attacks of arm pain without neurologic abnormalities (27). Clinicians treating children should be aware that spinal tumors can present with a pain component and use this in their diagnostic algorithms. It is important to perform a complete neurologic examination looking for central nervous system etiology and perform imaging studies where appropriate. Weakness and gait disturbance were found in more than 50% of these children. This emphasizes that a complete evaluation is warranted when children have atypical, unrelenting pain in the presence or absence of neurologic compromise.
The age of the child seems to have some impact on the tumor etiology. Very young children tend to have more developmental types of tumors including teratomas, dermoid tumors, and epidermoid tumors, whereas older children more commonly have astrocytomas.
Scoliosis can be a presenting issue in children with spinal cord tumors, as seen in 3 children in our study. However, many authors have found spinal column deformity after laminectomy and irradiation to be a serious long-term problem in children (28,29). In a retrospective review by Mottl and Koutecky of 9 children under 17 years of age with intramedullary tumors, 7 underwent laminectomy with subtotal resection, with all but 1 receiving postoperative radiation therapy (28). Of the 5 survivors evaluated at a mean follow-up of 3 years 4 months, all required bracing for spinal deformity. Similarly, Papagelopoulos et al published a retrospective study about the occurrence of spinal column deformity or instability after multilevel lumbar or thoracolumbar total laminectomy for removal of benign intraspinal tumors in 12 children and adolescents (ages 17 or younger with mean age of 11 years) and 24 young adults (ages 18–30 y) (29). At a mean follow-up of 14 years, spinal column deformity occurred in 33% of children and adolescents and 8% of young adults, while spondylolisthesis occurred in 16.6% of children and adolescents and 8% of young adults. There was an increased incidence of postoperative spinal deformity in patients who had more than 2 laminae removed or facetectomy performed at the initial operation. Thus, routine and prolonged follow-up of these children and adolescents to monitor development of spinal deformities is imperative.
An important question related to children with spinal cord tumors and masses is the functional outcome. The documented predictors of function have been outlined in the literature as tumor type and prediagnosis function (19). The one functional scale used in the literature is the McCormick scale. We opted to use the same scale in determining outcomes of our study population. It uses motor and sensory parameters but does not address bowel or bladder function. Using this functional assessment, it is noted that most children had some type of motor or sensory disturbance on presentation. Our data, although somewhat different in tumor etiology, are fairly consistent with previous published data. For children whose assessment was grade 5, or were basically paraplegic/quadriplegic, there was no change in neurologic function. There were 3 children who became paraplegic after treatment, as reflected in the change in their McCormick grade. We did have several children who improved from grade 4 to a lower level. Long-term neurogenic bowel and bladder were noted in 23%, and of these it was most often noted in those with a neuroblastoma or astrocytoma.
CONCLUSIONS
Pediatric spinal cord tumors are a rare entity and account for a small percentage of tumors of the central nervous system. In this study population, the predominant location was intramedullary, which is consistent with previous reported series. Interestingly, in this study group, developmental tumors were the most common and were diagnosed by histology. These data deviate from other study populations' classification of pediatric spinal cord tumors. We were very interested in evaluating functional outcomes of tumors in pediatric cases. The McCormick scale provided a system to grossly categorize mobility and sensory status both pre- and posttreatment. Children with the worst outcomes (grade 5) were most often categorized as having an astrocytoma or neuroblastoma. This initial review and these data provide a starting point for further prospective studies, particularly emphasizing a more in-depth assessment of outcomes, presenting symptoms and association with tumors.
REFERENCES
- Hardison HH, Packer RJ, Rorke LB, Schut L, Sutton LN, Bruce DA. Outcome of children with primary intramedullary spinal cord tumors. Childs Nerv Syst. 1987;3:89–92. doi: 10.1007/BF00271131. [DOI] [PubMed] [Google Scholar]
- Stiller CA, Nectoux J. International incidence of childhood brain and spinal tumours. Int J Epidemiol. 1994;23:458–464. doi: 10.1093/ije/23.3.458. [DOI] [PubMed] [Google Scholar]
- Nadkarni TD, Rekate HL. Pediatric intramedullary spinal cord tumors. Critical review of the literature. Childs Nerv Syst. 1999;15:17–28. doi: 10.1007/s003810050321. [DOI] [PubMed] [Google Scholar]
- Constantini S, Epstein F. Pediatric intraspinal tumors. In: Choux M, Di Rocco E, Hockley A, Walker M, editors. Pediatric Neurosurgery. London, UK: Churchill Livingstone; 1999. pp. 601–602. [Google Scholar]
- Schick U, Marquardt G. Pediatric spinal tumors. Pediatr Neurosurg. 2001;35:120–127. doi: 10.1159/000050404. [DOI] [PubMed] [Google Scholar]
- Rorke LB, Gilles FH, Davis RL, Becker LE. Revision of the World Health Organization classification of brain tumors for childhood brain tumors. Cancer. 1985;56(Suppl 7):1869–1886. doi: 10.1002/1097-0142(19851001)56:7+<1869::aid-cncr2820561330>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Cochrane DD, Poskitt K. Intramedullary spinal cord tumors in children. Neurosurg Clin N Am. 1992;3:931–945. [PubMed] [Google Scholar]
- Kumar R, Singh V. Benign intradural extramedullary masses in children of northern India. Pediatr Neurosurg. 2005;41:22–28. doi: 10.1159/000084861. [DOI] [PubMed] [Google Scholar]
- Townsend N, Handler M, Fleitz J, Foreman N. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004;43:629–632. doi: 10.1002/pbc.20082. [DOI] [PubMed] [Google Scholar]
- Auguste KI, Gupta N. Pediatric intramedullary spinal cord tumors. Neurosurg Clin N Am. 2006;17:51–61. doi: 10.1016/j.nec.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Patel MR, Tse V. Diagnosis and staging of brain tumors. Semin Roentgenol. 2004;39:347–360. doi: 10.1016/j.ro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bhatoe HS, Singh P, Chaturvedi A, Sahai K, Dutta V, Sahoo PK. Nondysraphic intramedullary spinal cord lipomas: a review. Neurosurg Focus. 2005;18(2):ECP1. doi: 10.3171/foc.2005.18.2.10. [DOI] [PubMed] [Google Scholar]
- Blount JP, Elton S. Spinal lipomas. Neurosurg Focus. 2001;10(1):e3. doi: 10.3171/foc.2001.10.1.4. [DOI] [PubMed] [Google Scholar]
- Schroeder S, Lackner K, Weiand G. Lumbosacral intradural lipoma. J Comput Assist Tomogr. 1981;5:274. doi: 10.1097/00004728-198104000-00029. [DOI] [PubMed] [Google Scholar]
- Marina N. Biology and therapy of pediatric malignant solid tumors. Cancer Chemother Biol Response Modif. 1999;18:550–589. [PubMed] [Google Scholar]
- Constantini S, Houten J, Miller DC, et al. Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg. 1996;85:1036–1043. doi: 10.3171/jns.1996.85.6.1036. [DOI] [PubMed] [Google Scholar]
- Jallo GI, Freed D, Epstein F. Intramedullary spinal cord tumors in children. Childs Nerv Syst. 2003;19:641–649. doi: 10.1007/s00381-003-0820-3. [DOI] [PubMed] [Google Scholar]
- Houten JK, Weiner HL. Pediatric intramedullary spinal cord tumors: special considerations. J Neurooncol. 2000;47:225–230. doi: 10.1023/a:1006418506213. [DOI] [PubMed] [Google Scholar]
- Sandalcioglu IE, Gasser T, Asgari S, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994;35:69–74. doi: 10.1227/00006123-199407000-00011. discussion 76. [DOI] [PubMed] [Google Scholar]
- Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. discussion 1143. [DOI] [PubMed] [Google Scholar]
- McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- Greenwood J., Jr. Surgical removal of intramedullary tumors. J Neurosurg. 1967;26:276–282. doi: 10.3171/jns.1967.26.2.0276. [DOI] [PubMed] [Google Scholar]
- Malis LI. Intramedullary spinal cord tumors. Clin Neurosurg. 1978;25:512–539. doi: 10.1093/neurosurgery/25.cn_suppl_1.512. [DOI] [PubMed] [Google Scholar]
- Kumar R, Singh V. Intramedullary mass lesion of the spinal cord in children of a developing milieu. Pediatr Neurosurg. 2004;40:16–22. doi: 10.1159/000076572. [DOI] [PubMed] [Google Scholar]
- Albright AL. Pediatric intramedullary spinal cord tumors. Childs Nerv Syst. 1999;15:436–438. doi: 10.1007/s003810050431. [DOI] [PubMed] [Google Scholar]
- Robertson PL. Atypical presentations of spinal cord tumors in children. J Child Neurol. 1992;7:360–363. doi: 10.1177/088307389200700405. [DOI] [PubMed] [Google Scholar]
- Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293–295. doi: 10.1002/(sici)1096-911x(199710)29:4<293::aid-mpo10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Papagelopoulos PJ, Peterson HA, Ebersold MJ, Emmanuel PR, Choudhury SN, Quast LM. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine. 1997;22:442–451. doi: 10.1097/00007632-199702150-00019. [DOI] [PubMed] [Google Scholar]