Abstract
Objective:
To determine whether persons who incur a spinal cord injury as children are at increased risk of mortality compared with persons injured as adults given comparable current age, sex, and injury severity.
Methods:
A total of 25,340 persons admitted to the National Spinal Cord Injury Statistical Center database or the National Shriners Spinal Cord Injury database who were not ventilator dependent and who survived more than 2 years after injury were included in this study. These persons contributed 274,020 person-years of data, with 3,844 deaths, over the 1973–2004 study period. Data were analyzed using pooled repeated observations analysis of person-years. For each person-year the outcome variable was survival/mortality, and the explanatory variables included current age, sex, race, cause of injury, severity of injury, and age at injury (the focus of the current analysis).
Results:
Other factors being equal, persons who were less than 16 years of age at time of injury had a 31% (95% CI = 3%–65%) increase in the annual odds of dying compared with persons injured at older ages (P = 0.013). This increased risk did not vary significantly by current age, sex, race, injury severity, or era of injury (P > 0.05).
Conclusion:
Life expectancy for persons injured as children appears to be slightly lower than that of otherwise comparably injured persons who suffered their injuries as adults. Nonetheless, persons who are injured young can enjoy relatively long life expectancies, ranging from approximately 83% of normal life expectancy for persons with minimal deficit incomplete injuries to approximately 50% of normal in high-cervical-level injuries without ventilator dependence.
Keywords: Spinal cord injuries, Life expectancy, Mortality, Epidemiology, Pediatrics, Logistic regression, Person-years, National Spinal Cord Injury Statistical Center
INTRODUCTION
Survival after spinal cord injury (SCI) has been extensively studied, and a large body of literature from around the world is available (1–19). Much is known about the factors affecting survival and the magnitude of their effect. One factor that has not been adequately studied, however, is the effect of being injured as a child. Longer duration of injury for comparable current age for those injured during childhood may increase mortality because of the prolonged period of exposure to both SCI-related complications and general health issues related to the relatively sedentary SCI lifestyle. People injured as children are at risk for the same complications as those with adult-onset SCI. Additionally, pediatric SCI has unique complications, such as scoliosis, which may affect survival because of its effect on pulmonary function and seating, thereby increasing risk of respiratory complications and pressure ulcers (20–28). Therefore, the purpose of the present study was to determine whether injury during childhood is a significant predictor of long-term survival after SCI. It is hypothesized that adults with SCI who were injured as children are subject to higher annual mortality rates than persons who were injured as adults, given comparable current age, gender, race, cause of injury, and injury severity.
METHODS
Study Population
The study population included a consecutive series of persons with traumatic SCI who were treated at any of 25 model SCI systems or 3 Shriners Hospitals for Children SCI units located throughout the United States. All data were submitted to the National Spinal Cord Injury Statistical Center (NSCISC) for inclusion in either the model system NSCISC database or a comparable National Shriners SCI (NSSCI) database.
Detailed descriptions of the history, eligibility criteria, data collection protocol, data quality control procedures, and current status of the NSCISC database have been published previously (29,30). This is the largest and most comprehensive source of data on SCI available. The database for survival studies, such as the one used in the present study, is larger still, as it has somewhat less restrictive eligibility criteria (3–6) than the NSCISC database does. Briefly, to be included individuals must have had a clinically discernible degree of neurologic (spinal cord) impairment after a traumatic event and been treated at a model SCI system.
To be included in the NSSCI database, individuals must have (a) had a clinically discernible degree of neurologic (spinal cord) impairment after a traumatic event (nontraumatic cases in the NSSCI database were excluded from this study), (b) been treated at a Shriners Hospitals for Children SCI unit, and (c) been 21 years of age or younger at the time of treatment. Data collection and quality control procedures for the NSSCI database are similar to those of the NSCISC database (31).
For purposes of this study, the NSCISC survival database and the NSSCI database were merged, and duplicate patients were removed based on either matching Social Security numbers or matching names and dates of injury. As persons who were ventilator-dependent (defined as requiring partial or total respiratory mechanical support on a long-term daily basis) made up less than 1% of the population and will be reported on elsewhere, they were excluded from the final data set used for this study.
Data Collection
Date of death was reported to the NSCISC by the data collectors at each hospital whenever, in the course of their routine follow-up data collection activities, they determined an individual to be deceased. In addition, during March and April 2004 the staff of the NSCISC checked on the survival status of persons in both databases by searching the Social Security Death Index (SSDI) online at www.ancestry.com. Persons not reported as deceased by the model system or Shriners Hospitals for Children SCI unit and not found in the SSDI were assumed alive on January 1, 2004. The SSDI has previously been found to be 92.4% sensitive and 99.5% specific for persons in the NSCISC database (6). Sensitivity and specificity of the SSDI for children in the NSSCI database has not been formally tested.
Factors Related to Survival
The following items were investigated as possible predictors of mortality:
Level and grade of spinal cord injury. The neurologic examination was performed just prior to model system discharge in accordance with the most current version of the International Standards for Neurological Classification of SCI (32). Neurologic level of injury was defined as the most caudal segment of the spinal cord with normal sensory and motor function on both sides of the body. The American Spinal Injury Association (ASIA) Impairment Scale was used to categorize the extent of injury as either neurologically complete (Grade A), incomplete with sensory but not motor function preserved through the S4-S5 segment (Grade B), incomplete with motor function preserved and more than half of key muscles below the neurologic level having a grade less than 3 out of 5 (Grade C), incomplete with motor function preserved and at least half of key muscles below the neurologic level having a grade of at least 3 out of 5 (Grade D), or normal motor and sensory function (Grade E).
Current age.
Sex.
Ethnicity. A white/nonwhite classification, as used in previous work (3,4), was investigated.
Etiology of injury. We investigated various causes, including motor vehicle accidents, sporting accidents, and gunshot wounds. As noted elsewhere (3), an etiology of violence tends to be associated with poorer socioeconomic class and nonwhite ethnicity.
Time since injury. The number of years postinjury for each person-year of observation that was used in the analysis.
Era of injury and current calendar year were each broken into decades, beginning with 1930. The latter is the subject of a separate study, which demonstrated that, after the first 3 years post-injury, there was no secular trend; ie, no effect of current calendar year on survival (33).
Age at injury.
Various interactions with age at injury. As the objective of this study was to examine whether those injured as children have higher subsequent mortality in adulthood compared to those injured as adults, additional analyses were conducted to determine whether the impact of young age at injury on the likelihood of mortality differed by current age group, era of injury, sex, and level/grade of injury. That is, analyses were performed to determine whether the main effect of young age at injury on mortality likelihood was modified by other (confounding) factors.
Statistical Methods
The pooled repeated observations method was used for analyzing the data (10). Here the unit of analysis is not a person, as in many survival analyses, but instead a person-year. In the person-year data set, each person-year is associated with a binary variable indicating whether the person lived or died during that year, and also with the set of possible explanatory variables (the factors listed above). Logistic regression analysis (34) was used to determine which of the factors were associated with survival. This approach has been widely used in related work (1,35–37). The use of logistic regression makes separation of the effects of calendar time, current age, and time postinjury easier than use of a cohort approach such as proportional hazards modeling (38). Given that some persons were admitted to the model SCI system or Shriners Hospital for Children SCI unit only after many years had elapsed postinjury, and to avoid the bias that would be caused by these delayed admissions, only person-years of observation that occurred after admission were included in the analyses. As a result, only calendar years from 1970 through 2004 were included in the analyses (even though the injury may have been prior to 1970). We further restricted attention to person-years of observation that were greater than 2 years postinjury so as to avoid the well-documented high mortality in the years immediately following the injury (3–6).
Model selection was carried out using Wald and deviance statistics for nested models, and the Akaike information criterion otherwise (10,34,35,38,39). The final logistic regression model was used to compute the annual probabilities of death for two cases: males with nonviolent etiology who survived 3 or more years postinjury and had (a) C5A or (b) paraplegia BC (T1-S5BC) injuries. These probabilities were converted to mortality rates for ages 5 to 75 years. At ages 76 and older, a separate model was used (19). The entire schedule of rates was used to construct a standard life table (40,41), from which the life expectancies (average number of additional years of life in a large group of similar persons) were obtained. Figures for the age- and sex-matched general population (42) are shown for comparison.
RESULTS
After exclusion of data on (a) postinjury follow-up years 1 and 2, (b) all ventilator-dependent persons, and (c) calendar years prior to either model SCI system or Shriners Hospitals for Children SCI unit admission, the final study population included 25,340 persons and 274,020 person-years of follow-up. There were 3,844 deaths during the study period. The average age at injury was 32 years.
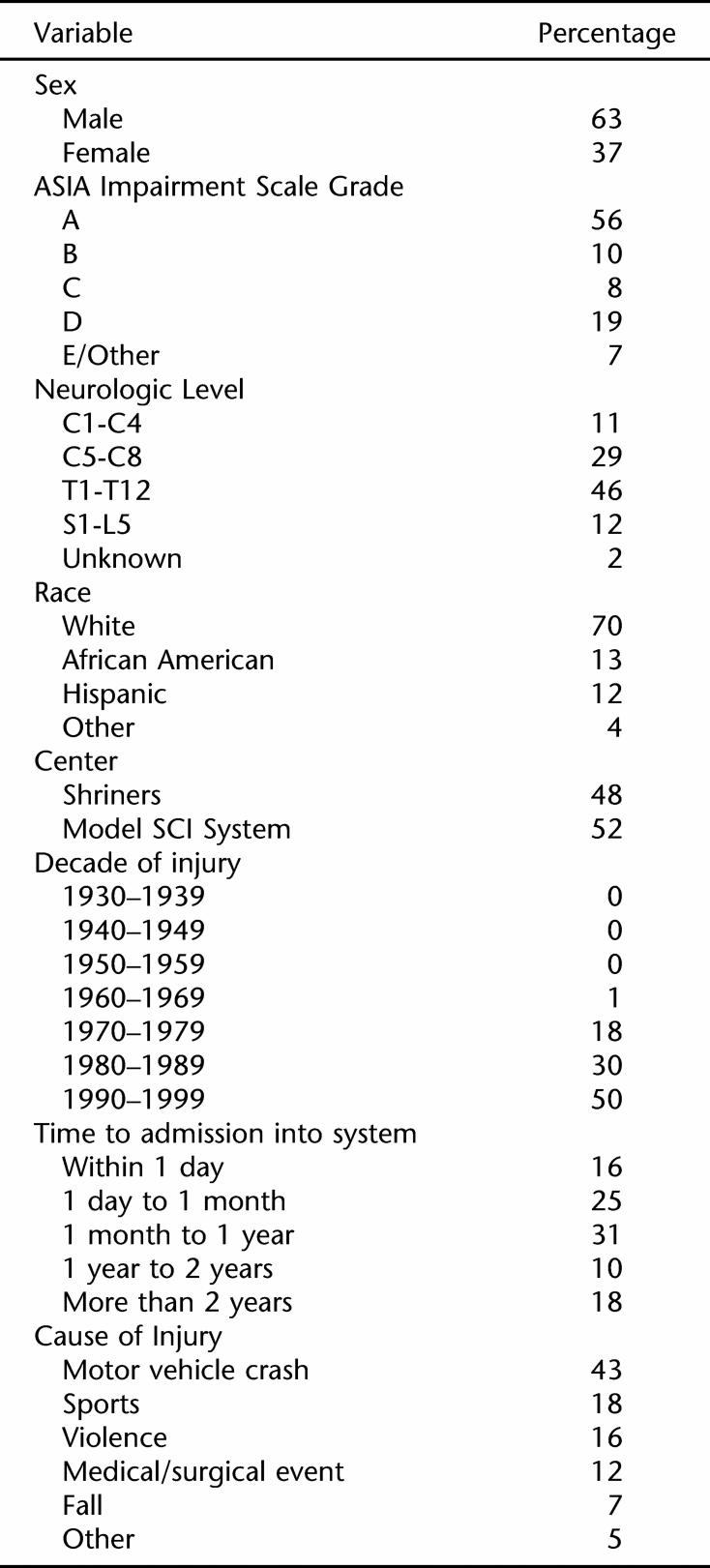
Table 1 shows some demographic and injury characteristics for the 2,373 persons injured under age 16 years who were included in the study. As indicated previously, this subpopulation was the primary focus group of this study. A thorough epidemiologic description of the childhood SCI population was recently published (31), and the interested reader is referred there for further details.
Table 1.
Characteristics of 2,373 Persons Injured Before Age 16 Years Who Were Not Ventilator Dependent
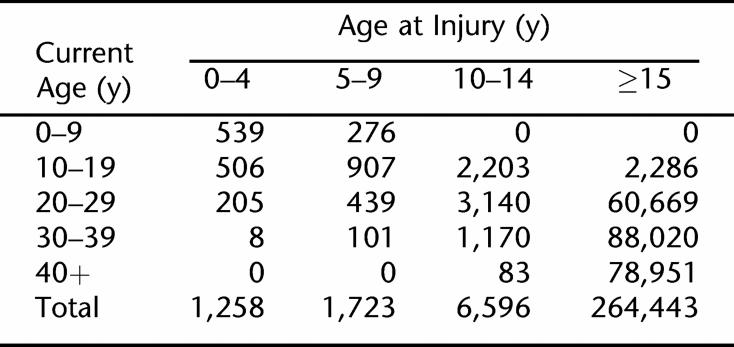
Table 2 shows the distribution of person-years by current age and age at injury. As expected, the preponderance of data is from persons injured at ages 15 years and older. Nevertheless, there are sufficient data on those injured as children to investigate the impact of injury at a young age on long-term mortality.
Table 2.
Distribution of Person-years by Current Age and Age at Injury
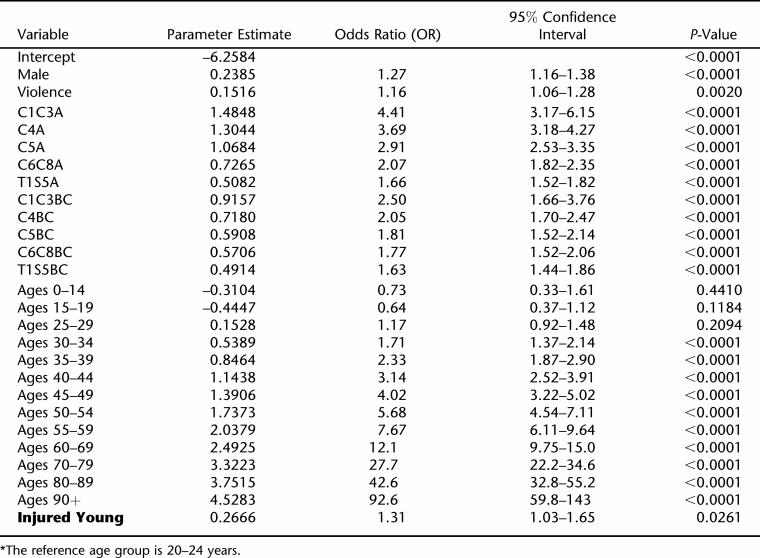
Based on the logistic regression modeling of person-years, the following factors were significantly associated with survival: current age, sex, etiology of injury (violence), and level/grade of injury (P < 0.05). Levels were found to be best categorized into 5 groups: high tetraplegia (C1–C3), mid tetraplegia (C4 and C5, separately), low tetraplegia (C6–C8), and paraplegia (T1-S5). Grade was best divided into 3 groups: A, B/C, and D/E. The 5 level-specific categories of grade D/E were not significantly different, and were thus combined, making 11 distinct groups. Five- or 10-year age groups were created, with age 20 to 24 years used as the reference group. Personal violence was the only etiology of injury that had significant predictive value. All other causes were therefore combined into a single reference group. Time since injury did not have an effect on survival after 2 years. We shall return to this finding in the Discussion. Race and decade of injury did not have a statistically or practically significant effect once the above factors were considered (P > 0.05) and were thus dropped from further consideration. We did not find any statistically significant interactions among the factors. The final logistic regression model is shown in Table 3.
Table 3.
Logistic Regression Model of the Yearly Probability of Dying*
There was no statistically significant difference in survival between those injured at ages 0–4, 5–9, and 10–15 years (P > 0.05), and there did not appear to be a trend with age, but the data were relatively sparse in the first 2 age groups and thus those specific comparisons had low statistical power. The 3 age-at-injury groups were therefore combined. The composite group had a 31% increased likelihood of dying in any given year following injury (odds ratio = 1.31, 95% CI = 1.03–1.65; see Table 3). The P-values shown in Table 3 are for 2-sided hypothesis testing. As our null hypothesis here was that persons injured young are at a higher risk, a 1-sided test, the P-value for our comparison is 0.026/2 = 0.013.
There were no statistically or practically significant interactions between young age at injury and any of the other prognostic factors (P > 0.05). That is, the effect of young age at injury did not appear to vary by current age, gender, race, cause of injury, level of injury, grade of injury, time since injury, or era of injury.
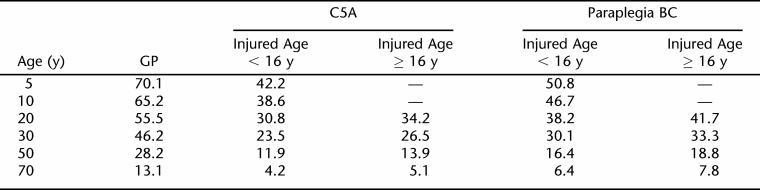
The model of Table 3 was used to compute life expectancies for 2 common cases: males with C5A or paraplegia BC (T1-S5BC) lesions. Table 4 shows these life-expectancies along with those of the general population. As can be seen, the odds ratio of 1.31 — 31% increased risk of death — has an appreciable effect on life expectancy. Life expectancies of persons injured young are several years lower than those of otherwise comparable persons who were injured at older ages. As expected, greater severity of impairment is also associated with lower life expectancies.
Table 4.
Life Expectancies for Males Who Are 2 or More Years Postinjury, With Nonviolent SCI Etiology
DISCUSSION
The results of this study confirm the hypothesis that persons injured as children have higher mortality as adults, compared with those injured as adults. This difference persists even after controlling for other factors (current age, gender, race, etiology of injury, time since injury, severity of injury, and decade of injury).
There are 2 possible alternative explanations for these findings. First, it might be suggested that the decade of injury accounts for the extra risk of mortality experienced by children, medicine being much better now than previously. However, decade of injury seems to affect survival only in the first few years after injury (3,10,33,43).
Second, it is possible that the difference is due to the long-term deleterious effects of a SCI. If this were true, a relationship between time since injury and the current mortality rate would be expected. However, as in previous studies (1,10,33,43), there did not appear to be such an effect: after the first 2 years postinjury, time since injury did not affect current survival.
Complications characteristic of pediatric-onset SCI may contribute to the extra morbidity and mortality in this group of patients. Nearly all children injured prior to skeletal maturity (roughly ages 11–13 years) will develop scoliosis, and approximately two thirds will experience scoliosis severe enough to require surgical correction (20–22). Pulmonary complications may be increased in individuals injured as children because of restrictive lung disease that occurs as a consequence of scoliosis (23). In addition, because of their neurologic deficits, children with SCI may be at greater risk for recurrent respiratory infections, which may affect pulmonary development in the growing child. Further, infants and young children with tetraplegia may be at risk of incipient respiratory failure, which may be manifested as sleep-disordered breathing (24). Hip dislocation is common among children injured prior to age 10 years and may result in uneven sitting that could increase the incidence of pressure ulcers (25–28). Lastly, there may be unknown effects of SCI on individuals injured as children, such as metabolic effects (metabolic syndrome), or issues related to internal organ development (kidneys or lungs that sustain infections during their growth for example), though there does not appear to be published evidence on these.
Limitations
The present study has several limitations. First, the NSCISC and NSSCI databases are not population based. They only capture data from approximately 13% to 15% of the persons who have sustained SCI in the US, and only from persons who are treated at model systems (29). It is possible that persons who are not treated at model systems experience different short-term and long-term mortality rates and that the effect of age at injury on mortality rates for those persons might be different than for persons who are treated at model systems.
Another limitation is that the NSCISC database does not include information on many factors such as smoking history, associated injuries, or pre-existing major medical conditions that might be of prognostic importance in determining life expectancy. However, it is unlikely that these confounding factors would lead to an overestimation of the effect of age at injury on mortality that was observed in this study.
As noted, the SSDI is 92.4% sensitive in identifying deceased persons with SCI. Although this was not the only method of identifying deceased persons, it is likely that a few persons who died were misclassified as alive, thereby causing a slight overestimation of life expectancy. In a recent study of deceased persons with traumatic brain injury, it was found that young age, being unmarried, and being female all significantly decreased the sensitivity of the SSDI (44). Therefore, both the underestimation of the mortality odds ratios and the overestimation of the life expectancy are likely to be greater for young persons, as well as persons whose injuries occurred at younger ages, than for older persons.
Further, there have been losses to follow-up in the two databases. This is particularly true for the NSSCI database, where the data collection protocol limits follow-up to persons whose current age is 21 or younger. When there was sufficient identifying information, these persons were followed after age 21 years through use of the SSDI, but this was not always possible. A previous study of losses to follow-up in the NSCISC database revealed that persons who were lost were more likely to have less severe injuries and be nonwhite (45). However, while these losses to follow-up might bias overall risk levels and life expectancy estimates, they are unlikely to bias the relationships between prognostic factors and mortality risk.
It might be expected that, other things being equal, mortality would be higher for those who have been injured for a very long time. For example, one might expect that a 60-year-old man injured at age 20 years would have a worse prognosis than one injured at age 50 years. The former would have a longer time to develop various SCI complications (such as pressure ulcers and urinary tract infections), as well as increased complications of aging (cardiovascular and metabolic) as a result of a relatively sedentary lifestyle. Somewhat surprisingly, we did not observe such an effect. It may be, however, that the length of follow-up — at most 35 years — was not sufficient to examine this hypothesis.
Finally, the definition of young age at injury that was used in this study — age 15 years or less — is rather arbitrary. It is unlikely that there is a large change from ages 14 to 16 years at injury. Rather, one would expect a gradient: the younger the age at injury, the higher the mortality odds ratio, though we did not find sufficient evidence of such. The limited sample sizes at young injury ages, however, precluded further evaluation of any possible gradient for age at injury and mortality risk.
CONCLUSIONS
Injury at a young age portends higher mortality and, thus, lower life expectancy, than does injury sustained during adulthood. The reasons for this are not well documented. Further detailed study of the various causes of morbidity and mortality in this population would be helpful.
Acknowledgments
This study was funded in part by grant number H133A011201 from the National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, US Department of Education, Washington, DC. Funding for the NSSCI database was provided by Shriners Hospitals for Children.
REFERENCES
- Krause JS, DeVivo MJ, Jackson AB. Health status, community integration, and economic risk factors for mortality after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1764–1773. doi: 10.1016/j.apmr.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Coll JR, Frankel HL, Charlifue SW, Whiteneck GG. Evaluating neurological group homogeneity in assessing the mortality risk for people with spinal cord injuries. Spinal Cord. 1998;36:275–279. doi: 10.1038/sj.sc.3100497. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL. Long-term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen Publishing; 1995. pp. 289–316. [Google Scholar]
- DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Seven-year survival following spinal cord injury. Arch Neurol. 1987;44:872–875. doi: 10.1001/archneur.1987.00520200074023. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL, Black KJ. Prognostic factors for 12-year survival after spinal cord injury. Arch Phys Med Rehabil. 1992;73:156–162. [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, et al. Long term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Strauss DJ, DeVivo MJ, Shavelle RM. Long-term mortality risk after spinal cord injury. J Insur Med. 2000;32:11–16. [Google Scholar]
- Yeo JD, Walsh J, Rutkowski S, Soden R, Craven M, Middleton J. Mortality following spinal cord injury. Spinal Cord. 1998;36:329–336. doi: 10.1038/sj.sc.3100628. [DOI] [PubMed] [Google Scholar]
- Strauss DJ, Shavelle RM, DeVivo MJ, Day S. An analytic method for longitudinal mortality studies. J Insur Med. 2000;32:217–225. [PubMed] [Google Scholar]
- Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F. Survival and cause of death after traumatic spinal cord injury: a long-term epidemiological survey from Denmark. Spinal Cord. 1997;35:76–85. doi: 10.1038/sj.sc.3100351. [DOI] [PubMed] [Google Scholar]
- O'Connor P. Survival after spinal cord injury in Australia. Arch Phys Med Rehabil. 2005;86:37–47. [PubMed] [Google Scholar]
- Geisler WO, Jousse AT, Wynne-Jones M, Breithaupt D. Survival in traumatic spinal cord injury. Paraplegia. 1983;21:364–373. doi: 10.1038/sc.1983.60. [DOI] [PubMed] [Google Scholar]
- Samsa GP, Patrick CH, Feussner JR. Long-term survival of veterans with traumatic spinal cord injury. Arch Neurol. 1993;50:909–914. doi: 10.1001/archneur.1993.00540090018005. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Ivie CS. Life expectancy of ventilator-dependent persons with spinal cord injuries. Chest. 1995;108:226–232. doi: 10.1378/chest.108.1.226. [DOI] [PubMed] [Google Scholar]
- Krause JS, Sternberg M, Maides J, Lottes S. Mortality after spinal cord injury: an 11-year prospective study. Arch Phys Med Rehabil. 1997;78:815–821. doi: 10.1016/s0003-9993(97)90193-3. [DOI] [PubMed] [Google Scholar]
- Catz A, Thaleisnik M, Fishel B, Ronen J, Spasser R, Fredman B, Shabtay E, Gepstein R. Survival following spinal cord injury in Israel. Spinal Cord. 2002;40:595–598. doi: 10.1038/sj.sc.3101391. [DOI] [PubMed] [Google Scholar]
- Imai K, Kadowaki T, Aizawa Y. Standardized indices of mortality among persons with spinal cord injury: accelerated aging process. Industrial Health. 2004;42:213–218. doi: 10.2486/indhealth.42.213. [DOI] [PubMed] [Google Scholar]
- Strauss DJ, Vachon PJ, Shavelle RM. Estimation of future mortality rates and life expectancy in chronic medical conditions. J Insur Med. 2005;37:20–34. [PubMed] [Google Scholar]
- Dearolf WW, Betz RR, Vogel LC, Levin J, Clancy MC, Steel HH. Scoliosis in pediatric spinal cord injured patients. J Pediatr Orthop. 1990;10:214–218. [PubMed] [Google Scholar]
- Bergstrom EM, Short DJ, Frankel HL, Henderson NJ, Jones PR. The effect of childhood spinal cord injury on skeletal development: a retrospective study. Spinal Cord. 1999;37:838–846. doi: 10.1038/sj.sc.3100928. [DOI] [PubMed] [Google Scholar]
- Apple DF, Jr, Anson CA, Hunter JD, Bell RB. Spinal cord injury in youth. Clin Pediatr. 1995;34:90–95. doi: 10.1177/000992289503400205. [DOI] [PubMed] [Google Scholar]
- Zaba R. Peak expiratory flow in children and adolescents with idiopathic scoliosis. Wiad Lek. 2003;56:552–555. [PubMed] [Google Scholar]
- Flavell H, Marshall R, Thornton AT, et al. Hypoxic episodes during sleep in high tetraplegia. Arch Phys Med Rehabil. 1992;73:623–627. [PubMed] [Google Scholar]
- Rink P, Miller F. Hip instability in spinal cord injury patients. J Pediatr Orthop. 1990;10:583–587. doi: 10.1097/01241398-199009000-00002. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Chafetz RS, Betz RR, Gaughan J. Incidence and degree of hip subluxation/dislocation in children with spinal cord injury. J Spinal Cord Med. 2004;27(Suppl 1):S80–S83. doi: 10.1080/10790268.2004.11753423. [DOI] [PubMed] [Google Scholar]
- Vogel LC, Krajci KA, Anderson CJ. Adults with pediatric-onset spinal cord injury: Part 2: Musculoskeletal and neurological complications. J Spinal Cord Med. 2002;25:117–123. doi: 10.1080/10790268.2002.11753611. [DOI] [PubMed] [Google Scholar]
- Garland DE, Shimoyama ST, Lugo C, Barras D, Gilgoff I. Spinal cord insults and heterotopic ossification in the pediatric population. Clin Orthop Relat Res. 1989;245:303–310. [PubMed] [Google Scholar]
- Stover SL, DeVivo MJ, Go BK. History, implementation, and current status of the national spinal cord injury database. Arch Phys Med Rehabil. 1999;80:1365–1371. doi: 10.1016/s0003-9993(99)90246-0. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Vogel LC. Epidemiology of spinal cord injury in children and adolescents. J Spinal Cord Med. 2004;27(Suppl):S4–S10. doi: 10.1080/10790268.2004.11753778. [DOI] [PubMed] [Google Scholar]
- American Spinal Injury Association . International Standards for Neurological Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 2002. revised 2002. [Google Scholar]
- Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Hosmer D, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- Cupples LA, D'Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1998;7:205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Modeling the days of our lives: Using survival analysis when designing and analyzing longitudinal studies of duration and the timing of events. Psychol Bull. 1991;110:268–290. [Google Scholar]
- Strauss DJ, Shavelle RM, Ashwal S. Life expectancy and median survival time in the permanent vegetative state. Pediatr Neurol. 1999;21:626–631. doi: 10.1016/s0887-8994(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Collett D. Modelling Survival Data in Medical Research. London: Chapman and Hall; 1994. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman and Hall; 1989. Chapter 2. [Google Scholar]
- Schoen R. Modelling Multigroup Populations. New York: Plenum Press; 1988. [Google Scholar]
- Anderson TW. Life Expectancy in Court: A Textbook for Doctors and Lawyers. Vancouver BC: Teviot Press; 2002. [Google Scholar]
- Arias E. Bethesda, MD: National Center for Health Statistics; 2004. United States Life Tables, 2001. National Vital Statistics Reports, Vol 52, No 14. [PubMed] [Google Scholar]
- Shavelle RM, DeVivo MJ, Strauss DJ, Paculdo DJ, Lammertse DP, Day SM. Long-term survival of persons ventilator dependent after spinal cord injury. J Spinal Cord Med. 2006;29:511–519. doi: 10.1080/10790268.2006.11753901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Underhill AT, Fine PR. Accuracy of world-wide-web death searches for persons with traumatic brain injury. Brain Inj. 2004;18:1155–1162. doi: 10.1080/02699050410001672323. [DOI] [PubMed] [Google Scholar]
- Richards JS, Go BK, Rutt RD, Lazarus PB. The national spinal cord injury collaborate database. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen; 1995. pp. 10–20. [Google Scholar]