Abstract
Objective:
To determine the age-specific incidence, prevalence, and characteristics of fractures in persons with spina bifida.
Design:
Year-long historical cross-sectional study.
Subjects:
Two hundred twenty-one consecutive patients aged 2–58 years evaluated in 2003 at a regional referral center. Twenty percent (n=44) were children age 2–10 years; 30% (n=68) were adolescents age 11–18 years; and 50% (n =109) were adults age 19– 58 years. Fifty-five percent (n =121) were female; 64% (n = 141) had shunted hydrocephalus. Fifty-eight percent (n = 129) were community ambulators. Defect levels included 14% (n=31) thoracic; 37% (n=81) mid-lumbar; 35% (n=79) low-lumbar; and 14% (n=30) sacral.
Methods:
Chart review of 221 consecutive children, adolescents, and adults enrolled in a spina bifida program in Syracuse, New York, was used to determine incidence and prevalence rates. Chi-square was used for subgroup analyses, and linear regression was used to examine independent association of motor level, functional independence (Functional Independence Measures score), body mass index (BMI), shunted hydrocephalus, epilepsy, and/or other congenital anomalies with fractures, controlling for insurance status, race/ethnicity, age, and sex.
Results:
Annual incidence of fractures among children, adolescents, and adults was 23/1000; 29/1000; and 18/1000, respectively. Overall prevalence was 200/1000. One in 4 patients with fractures reported multiple fractures. Median age at first fracture was 11 years. Most fractures involved the femur or tibia. Comparisons between adult- and childhood-onset fractures were not significant for difference in sex, BMI, defect level, functional independence, shunted hydrocephalus, epilepsy, or other congenital anomalies. In regression models only defect level RR =1.646 (P =0.019; 95% CI 1.085–2.498) and age RR =1.033 (P =0.036; 95% CI 1.002–1.065) were independently associated with fractures.
Conclusions:
Fractures in persons with spina bifida are most common during early adolescence. Environmental modifications may be more effective than pharmacological treatment in reducing the prevalence of fractures in this population.
Keywords: Spina bifida, Fracture, Child, Adolescence, Adult, Bone loss
INTRODUCTION
Fractures occur commonly in children with spina bifida, with reported prevalence around 30%. Fractures usually involve the metaphysis or diaphysis of insensate lower extremities (1–3). Physeal fractures are described but are less common (<4%) (4–6). Fractures associated with immobilization after hip or spine surgery have also been described (7,8). Risk factors that predispose children with spina bifida to fractures include higher defect level (thoracic > lumbar > sacral), decreased sensation, osteopenia, and nonambulatory status (3,9,10). Little is known about fracture risk in adults with spina bifida. Dual-energy x-ray absorptiometry (DEXA) studies typically document osteopenia in individuals with spina bifida (11). The clinical implications of this, however, are unknown. There is also no clear consensus on whether to treat adults with spina bifida who are at risk for fractures with bisphosphonates, and, specifically, whether they are at increased risk for esophagitis or other adverse effects of treatment because of body habitus and/or Chiari-related oromotor dysfunction (12). Information on fracture rates may help guide clinicians who care for these patients.
Fractures are also common in individuals with spinal cord injury. In a population-based study, the crude fracture rate for patients with spinal cord injury was 2% per year. Those with lumbar lesions had more fractures than did those with cervical lesions (13). These data support the notion that environmental factors related to activity contribute to fracture rates in this population. Physiologic factors have also been identified. DEXA studies have documented that demineralization occurs below the level of the spinal cord injury and more commonly in weight-bearing skeletal areas such as the distal femur and proximal tibia (14). These sites correlate with common fracture locations for this population. Female sex and menopausal status are also associated with increased fracture risk (15). There are no published data on fracture risk across the lifespan for children, adolescents, and adults with spinal cord injury.
We hypothesized that fractures are a significant pediatric concern in persons with spina bifida and that predisposing risk factors in this population, as with spinal cord injury, are best understood both in terms of environmental interaction and as intrinsic to the patient. Children with executive dysfunction and/or who lack experience typically begin to participate in unsupervised activities during late childhood/early adolescence and may, therefore, be prone to fractures at this time. Adults with spina bifida may be protected from fractures because of better transfer technique, obesity, and/or environmental factors that result in a sedentary lifestyle. Thus our central hypothesis was that incidence and prevalence figures across the lifespan would reflect higher fracture rates among children than among adults.
The objectives of this study were threefold: (a) to determine incidence and prevalence rates of fractures in children, adolescents, and adults with spina bifida; (b) to describe characteristics of fractures for each age group; and (c) to determine whether distinct risk factors occurred for each age group that could inform age-specific prevention strategies.
METHODS
Study Design
We conducted a cross-sectional historical study by chart review. The study protocol was approved by the Research Subjects Review Board at SUNY Upstate Medical University.
Subjects and Setting
Subjects were 221 consecutive individual patients aged 2 to 58 years who were evaluated for routine comprehensive care during the 2003 calendar year at the Spina Bifida Center of Central New York. Our center is a state-funded hospital-based clinic located in an urban setting at SUNY Upstate Medical University in Syracuse, New York. It serves a 24-county catchment area that includes several small cities and an extensive rural population. The center has offered comprehensive care to patients with spina bifida for more than 30 years. It is staffed by a neurodevelopmental pediatrician, 2 nurse specialists, a physical therapist, an occupational therapist, and a social worker. In addition, the center provides on-site consultation with 2 urologists, a physiatrist, and an orthopedic surgeon.
Measures
We used patient reports of fractures at annual comprehensive visit and chart documentation of previous fractures for primary outcome data. Demographic data were extracted from billing sheets. Information regarding past medical and surgical history was extracted from the patient problem list and correspondence sections of the patient charts. Archived charts were also reviewed. Functional Independence Measures (FIM) were obtained routinely on all adult patients by an occupational therapist with formal training in this standardized instrument (16). Body mass index (BMI) was calculated using crown-to-heel measurements for ambulators and arm span measurements for nonambulators. Arm span measurements were multiplied by 0.90 for those with thoracic functional motor level and by 0.95 for those with a mid-lumbar functional motor level (17,18).
Analyses
We used SPSS statistical software (version 11 for Windows) for all analyses. Descriptive statistics were used to determine incidence and prevalence rates for spontaneous fractures in 3 age groups. Chi-square statistics were used to examine differences in proportions among subgroups. Multiple linear regression models were developed to examine the independent association of two broad categories of risk with the outcome of interest: (a) Spina bifida–specific risk factors included defect level, ambulatory status, functional independence (FIM cognitive subscore), shunted hydrocephalus, seizure disorder, prolonged immobilization, presence of other congenital anomalies, and precocious puberty; and (b) General risk factors included age, sex, race/ethnicity, and BMI.
RESULTS
Sample characteristics: 221 patients aged 2 to 58 years were evaluated. Age range followed a normal distribution pattern. Median age was 19 years. Twenty percent (n = 44) were children aged 2–10 years; 30% (n = 68) were adolescents aged 11–18 years; and 50% (n = 109) were adults aged 19–58 years. Fifty-five percent were female. Race was predominantly Caucasian (93%). Only 2 patients reported Hispanic ethnicity. Forty-four percent of patients were publicly insured. Functional motor levels in our patient population included: thoracic/high lumbar (T/L2–3) 31; mid-lumbar (L3) 80; low-lumbar (L4–5) 79; sacral 30. Fifty-nine percent (n = 130) were community ambulators. Median cognitive subscore on the Functional Independence Measure was 27 on a 35-point scale. Sixty-four percent (n = 141) had shunted hydrocephalus. Twenty percent (n = 44) had a documented history of prolonged immobilization with spica casting and/or for scoliosis surgery. Ten percent (n = 22) were on anticonvulsants for epilepsy, with 7 of these having had documented seizures during the study year. Eight percent (n = 17) had other congenital anomalies. This included a heterogeneous group of diagnoses such as renal dysplasia, cleft palate, omphalocele, sensorineural hearing loss, VATER association, velocardiofacial syndrome, Klinefelter syndrome, Mohr syndrome, and Turner syndrome. Precocious puberty was documented in 2 female patients (1%). Prevalence of BMI less than third percentile (underweight) was 10% for children, 14% for adolescents, and 7% for adults. Prevalence of BMI for age at or greater than 85th percentile (overweight/obese) was 29% among children, 22% among adolescents, and 42% among adults.
Prevalence and Incidence
Forty-two fractures were documented for 221 patients over 4,844 patient years. Prevalence of fractures among all patients was 200/1000. The crude fracture rate was 9/1000 per patient year. Five fractures were reported during the study year. Thus, the annual incidence of fractures per clinic year among children, adolescents, and adults in this study was 23/1000, 29/1000, and 18/1000, respectively.
Characteristics
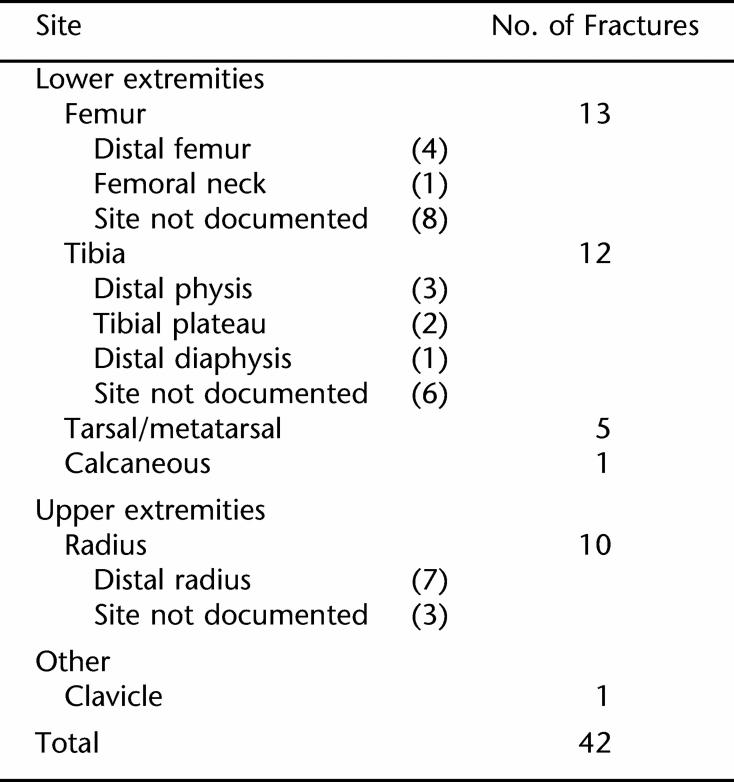
Most fractures involved the tibia and femur. Three fractures in our sample were physeal fractures. There were no vertebral or hip fractures. Review of problem list, operative notes, and archived medical reports revealed that 3 tibia fractures and 1 femur fracture occurred as perioperative complications of lower extremity surgeries. There was no documentation of perioperative fractures subsequent to hip or spine surgery. Two femur fractures occurred in the neonatal period due to birth injury and/or as an iatrogenic complication of myelomeningocele repair. Thus nearly one third (12/42) of fractures in this chart review study were postoperative neuropathic fractures.
Table 1 summarizes fracture sites for the 42 patients with fractures in this study. Fractures were more common in children than adults. Median age at first fracture was 11 years. Among children and adolescents, the mechanism of injury often could not clearly be established. Of note, none of the pediatric fractures that occurred during this year-long study were witnessed by an adult. A first fracture was documented in adulthood for 7 patients. Fractures among adults were typically attributed to accidental falls (commonly in the bathroom) or during exercise. Nearly 1 in 4 patients (9 men and 2 women) experienced multiple fractures. All individuals in this subgroup were nonambulatory and with higher defect levels (thoracic 2; mid-lumbar 7; low-lumbar 2). Table 2 lists the site and mechanism of injury for fractures that occurred among children, adolescents, and adults during the study year.
Table 1.
Fracture Sites
Table 2.
Fractures That Occurred During the Study Year
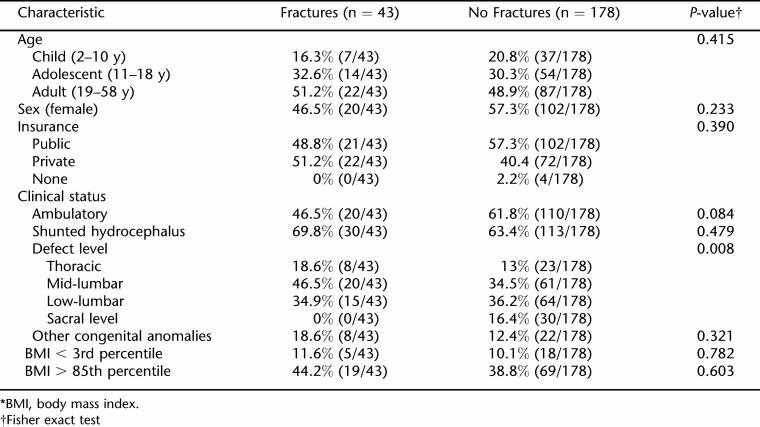
Comparisons between subgroups with and without fractures are listed on Table 3. Chi-square analyses documented a significant difference in proportions among defect level between these 2 groups (P = 0.008). Chi-square tests comparing patients with adult-onset vs childhood-onset fractures were not significant for sex, BMI, functional motor level, cognitive subscore on the FIM, presence of shunt, seizure disorder, or other congenital anomalies. Chi-square tests comparing fracture rates among subgroups of adults who were underweight and overweight also were not significant.
Table 3.
Characteristics Found in Fracture vs No Fractures Groups*
In regression models, only defect level RR = 1.646 (P = 0.019; 95% CI 1.085–2.498) and age RR = 1.033 (P = 0.036; 95% CI 1.002–1.065) were independently associated with fractures. Twenty-six percent (8/31) of patients with a thoracic defect level had experienced a fracture. This compared with 25% (20/81) for patients with a mid-lumbar motor level and 19% (15/79) among patients with low-lumbar functional motor levels. None of the patients with sacral defect levels had experienced a fracture. Regression models did not identify an independent association between BMI and fracture rates.
DISCUSSION
Our study documents that fractures are more common in adolescents than adults with spina bifida. Fracture sites and fracture characteristics appear to be similar. The only risk factors for fractures that we were able to identify were younger age and higher defect level. We had insufficient data to comment on specific environmental risk factors, particularly among children, many of whom had fractures that were not witnessed and for which the mechanism thus was not established.
Although shunted hydrocephalus and cognitive sub-scores on the FIM were not associated with fractures, the median age at first fracture (11 y) suggests that inexperience and/or poor judgment during physical activity may contribute to increased fracture risk for young adolescents as they gain independence and receive less supervision. Physiological factors such as changes in bone density during early adolescence may also play a role (19). Interestingly, our study found no association between BMI and fracture risk. We had hypothesized that higher BMI would be a protective factor. The high rates of obesity among adults and a relatively small sample size may have limited our ability to define an association.
Our study highlights similarities and differences in fracture rates and characteristics between individuals with paraplegia due to spinal cord injury vs paraplegia due to spina bifida. Higher defect level and adolescent age were risk factors identified in our study. For individuals with spinal cord injury, low bone mineral density, age, sex, duration after injury, muscle spasticity, and complete injury are identified risk factors (15).
Fracture sites are similar for both patient groups. Among individuals with spinal cord injury this has been correlated with regional bone loss in the long bones as measured by DEXA. Interestingly, vertebral bone mineral density is maintained or increased over time in this population (20). Our study did not examine bone mineral density. However, we documented a protective effect on vertebral fractures and a fracture distribution pattern among our patients with spina bifida that is strikingly similar to the spinal cord injury population.
Limitations
This study was conducted using a convenience sample. Chart review may not have captured all instances of fractures. We did not have the statistical power to eliminate effects of anticonvulsant use or other congenital anomalies on fracture rates. We also did not collect data, or examine fracture rates, in a subgroup of patients who were theoretically at risk for fractures because of metabolic acidosis related to ileal conduits or augmentation cystoplasty (21). Finally, we had very limited data on bone mineral density. Thus we cannot comment on the relationship between osteopenia and fractures in this study.
CONCLUSIONS
Environmental modifications during late childhood/early adolescence may decrease the occurrence of fractures in individuals who have spina bifida. A longitudinal cohort study with serial bone mineral density measurements would be necessary to further delineate factors associated with the development of osteoporosis and fractures in this population. A controlled trial of environmental modification for primary prevention of spontaneous fractures in children with spina bifida, such as group visits for safe transfer instruction, is also worth consideration.
Acknowledgments
The SUNY Upstate Medical University Department of Pediatrics Faculty Development Funds for database entry are gratefully acknowledged.
REFERENCES
- Drennan JC, Freehaffer AA. Fractures of the lower extremities in paraplegic children. Clin Orthop. 1971;77:211–217. [PubMed] [Google Scholar]
- Menelaus MB. The Orthopedic Management of Spina Bifida Cystica. New York, NY: Churchill Livingstone; 1980. [Google Scholar]
- Parsch K. Origin and treatment of fractures in spina bifida. Eur J Pediatr Surg. 1991;1:298–305. doi: 10.1055/s-2008-1042509. [DOI] [PubMed] [Google Scholar]
- Rodgers WB, Schwend RM, Jaramillo D, Kasser JR, Emans JB. Physeal widening in children with myelomeningocele. J Bone Joint Surg Br. 1989;71:30–32. doi: 10.1302/0301-620X.71B1.2915000. [DOI] [PubMed] [Google Scholar]
- Robert JA, Bennet GC, MacKenzie JR. Physeal widening in children with myelomeningocele. J Bone Joint Surg Br. 1989;71:30–32. doi: 10.1302/0301-620X.71B1.2915000. [DOI] [PubMed] [Google Scholar]
- Rodgers WB, Schwend RM, Jaramillo D, Kasser JR, Emans JB. Chronic physeal fractures in myelodysplasia: magnetic resonance analysis, histologic description, treatment, and outcome. J Pediatr Orthop. 1997;17:615–621. doi: 10.1097/00004694-199709000-00008. [DOI] [PubMed] [Google Scholar]
- Drummond DS, Moreau M, Cruess RL. Postoperative neuropathic fractures in patients with myelomeningocele. Dev Med Child Neurol. 1981;23:147–150. doi: 10.1111/j.1469-8749.1981.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lyne ED. Pathologic fractures in severely handicapped children and young adults. J Pediatr Orthop. 1990;10:497–500. [PubMed] [Google Scholar]
- Kumar SJ, Cowell HR, Townsend P. Physeal, metaphyseal, and diaphyseal injuries of the lower extremities in children with myelomeningocele. J Pediatr Orthop. 1984;4:25–27. doi: 10.1097/01241398-198401000-00006. [DOI] [PubMed] [Google Scholar]
- Reikeras O, Hellum C. Fractures in children with myelomeningocele. Arch Orthop Traumatic Surg. 1981;98:25–28. doi: 10.1007/BF00389706. [DOI] [PubMed] [Google Scholar]
- Quan A, Adams R, Ekmark E, Baum M. Bone mineral density in children with myelomeningocele. Pediatrics. 1998;102(3):e34. doi: 10.1542/peds.102.3.e34. [DOI] [PubMed] [Google Scholar]
- Liptak GS. Evidence-based Practice in Spina Bifida: Developing a Research Agenda. Washington, DC: Spina Bifida Association of America; 2004. [Google Scholar]
- Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physio-pathology to therapy. Spinal Cord. 2006;44:203–210. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporosis Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- Dodds TA, Matrin DP, Stolov WC, Deyo RA. A validation of the Functional Independence Measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Rosenblum MF, Finegold DN, Charney EB. Assessment of stature of children with myelomeningocele, and usefulness of arm-span measurement. Dev Med Child Neurol. 1983;25:338–342. doi: 10.1111/j.1469-8749.1983.tb13767.x. [DOI] [PubMed] [Google Scholar]
- Jarzem PF, Gledhill RB. Predicting height from arm measurements. J Pediatr Orthop. 1993;13:761–765. doi: 10.1097/01241398-199311000-00014. [DOI] [PubMed] [Google Scholar]
- Boot AM, de Ridder MAJ, Pols HAP, Krenning EP, de Muinck Keizer-Schrama SMPF. Bone mineral density in children and adolescents: relation to puberty, calcium intake, and physical activity. J Clin Endocrinol Metab. 1997;82:57–62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg. 2001;83A:1195–1200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Mingin GC, Nguyen HT, Mathia RS, et al. Growth and metabolic consequences of bladder augmentation in children with myelomeningocele and bladder exstrophy. Pediatrics. 2002;110:1193–1198. doi: 10.1542/peds.110.6.1193. [DOI] [PubMed] [Google Scholar]