Abstract
Objective:
The purpose of this study was to determine the prevalence of components of the metabolic syndrome in adolescents with spinal cord injury (SCI) and spina bifida (SB), and their associations with obesity in subjects with and without SCI and SB.
Methods:
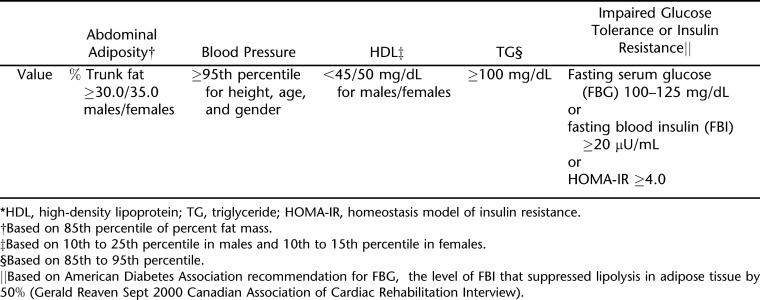
Fifty-four subjects (20 SCI and 34 SB) age 11 to 20 years with mobility impairments from lower extremity paraparesis were recruited from a hospital-based clinic. Sixty able-bodied subjects who were oversampled for obesity served as controls (CTRL). Subjects were categorized as obese if their percent trunk fat measured by dual x-ray absorptiometry (DXA) was >30.0% for males and >35.0% for females. Ten SCI, 24 SB, and 19 CTRL subjects were classified as obese. Fasting serum samples were collected to determine serum glucose, insulin, and lipid concentrations. Metabolic syndrome was defined as having ≥3 of the following components: (a) obesity; (b) high-density lipoprotein (HDL) <45 mg/dL for males; <50 mg/dL for females; (c) triglycerides ≥100 mg/dL; (d) systolic or diastolic blood pressure ≥95th percentile for age/height/gender, and (e) insulin resistance determined by either fasting serum glucose 100–125 mg/dL; fasting insulin ≥20μU/mL; or homeostasis model assessment of insulin resistance ≥4.0.
Results:
Metabolic syndrome was identified in 32.4% of the SB group and 55% of the SCI group. Metabolic syndrome occurred at a significantly higher frequency in obese subjects (SB = 45.8%, SCI = 100%, CTRL = 63.2%) than nonobese subjects (SB = 0%, SCI = 10%, CTRL = 2.4%).
Conclusions:
The prevalence of metabolic syndrome in adolescents with SB/SCI is quite high, particularly in obese individuals. These findings have important implications due to the known risks of cardiovascular diseases and diabetes mellitus associated with metabolic syndrome in adults, particularly those with spinal cord dysfunction.
Keywords: Spinal cord injuries, Insulin resistance, Metabolic syndrome, Adolescence, Obesity, Cardiovascular disease
INTRODUCTION
The prevalence of obesity has reached epidemic proportions worldwide (1–3). Obesity is caused by chronic positive energy balance due to increased energy intake and/or decreased energy expenditure/physical activity when combined with a genetic propensity for weight gain (4,5). Obesity in North American children and adolescents has tripled within the last twenty years (6), with a 45% increase in the United States since National Health and Nutrition Examination Survey (NHANES) III data were collected in 1994 (7). Based on NHANES 2003–2004, 17.1% of children and adolescents are ≥95th percentile Body Mass Index for age (6).
An important consequence of obesity is an increased prevalence of parameters associated with metabolic syndrome, a constellation of known risk factors for the development of type 2 diabetes (8–10), cardiovascular disease (10–13), and stroke (11,14,15). Metabolic syndrome has been shown to predict cardiovascular and coronary heart disease mortality (12,16,17). Obesity has been implicated as the primary inciting factor of the metabolic syndrome (18–20). Vanhala et al tracked 439 subjects from childhood to adulthood, and of the 30 adults who were identified with metabolic syndrome, 100% were obese as adults and 75% had been obese as children (21). Adolescent-onset obesity that persisted into adulthood increased the potential for lifelong problems, including increased severity of diseases associated with increased weight/adiposity (21).
The central features of metabolic syndrome are abdominal adiposity, hypertension, increased fasting glucose concentrations/abnormal glucose tolerance, and dyslipidemia characterized by increased serum triglycerides (TG), low serum high-density lipoprotein (HDL), and/or small dense low-density lipoprotein (LDL) particles (22). Insulin resistance as evidenced by hyper-insulinemia appears to be a key feature and may be an underlying/predisposing factor in the syndrome.
Adolescents with spina bifida (SB) or spinal cord injury (SCI) have limited mobility and physical activity, reduced total lean body mass, and reduced resting energy expenditure, leading to an increased risk for obesity. These patients typically have reduced lean body mass in the lower extremities, with increased abdominal and lower extremity fat tissue accumulation (23–31). More than 50% of children with SB have body mass index (BMI) values greater than the 95th percentile (32). Adults with SCI have been shown to have an increased prevalence of obesity (33,34); however, minimal research has been conducted to examine the relationship between obesity and increased risk factors for adverse health outcomes among adolescents with spinal cord dysfunction. Furthermore, paralysis was an exclusionary criterion for performing blood pressure measurements and collecting blood and urine samples in NHANES 2001–2002 (35).
Since obese adolescents have a greater prevalence of metabolic syndrome compared to nonobese adolescents and a greater risk of ultimately developing early onset of type 2 diabetes and cardiovascular disease, we hypothesized that adolescents with spinal cord dysfunction due to SB or SCI would exhibit a higher prevalence and severity of metabolic syndrome than able-bodied adolescents and that the prevalence of metabolic syndrome in these adolescents would be associated with their obesity status. We also sought to determine whether spinal cord dysfunction that occurs congenitally (SB) increased the incidence and severity of markers associated with metabolic syndrome compared to spinal cord dysfunction acquired as a consequence of a SCI years after birth.
METHODS
Study Population
Fifty-four adolescents who have spinal cord dysfunction with thoracic and upper lumbar paraplegia (34 due to SB and 20 due to SCI, all of whom were more than 1 y post injury) were recruited from specialty SB and SCI clinics at our institution. All SB and SCI patients at our clinic were asked to participate in this study. Although we used a convenience sample of adolescents with spinal cord dysfunction, the rate of obesity in our study is similar to the rates of obesity in the general population of adolescents with SCI and SB (36–39). Sixty able-bodied adolescents were recruited from the community through written advertisements and in person at the orientation session of the Fit Teen Program at the University of California Davis Medical Center prior to participating in their weight loss program. We oversampled obese controls (31.7% controls were obese compared to 17.1% in NHANES 2003–2004) to ensure groups of adequate size to detect an effect due to obesity compared to spinal cord dysfunction. Subjects were aged 11 to 20 years of age. Everyone who agreed to participate from the clinic, community, or Fit Teen program was included in the study unless excluded due to surgery within the past 4 months, cognitive impairment that would interfere with testing procedures, inability to understand instructions in English, known diabetes, medications for blood pressure or cholesterol management, or pregnancy. The Institutional Review Board for University of California at Davis and at Shriners Hospitals of Northern California for Children approved this study. Minors signed assent forms that explained the procedures and their rights before, during, and after the study. Written consent was also obtained from a parent or guardian. Testing was conducted in accordance with the approved protocol.
Procedures
The subjects visited the laboratory once after 12 hours of fasting. They were instructed to avoid unusual physical activity for the 24 hours prior to testing. Testing began between 7 AM and 9 AM. Height or length was measured to determine stature. Weight, waist circumference, and percentage of trunk fat by DXA (Hologic QDR4500A, version 11.2.1 Whole Body, Hologic, Bedford, MA) (40) were measured as indices of obesity. The spine itself was excluded from all scans so that subjects with and without hardware would be compared equally. Waist circumference was measured with a tape measure, snug without binding, parallel to the ground, at the umbilicus. Blood pressure was measured twice, with the appropriate pediatric or adult size cuff fitted on the right arm, while the subject was seated, after 30 minutes of rest. Height, BMI, and blood pressure values were standardized to z-scores to enable comparisons among different age groups. BMI z-scores were calculated based on the LMS formula proposed by Cole et al (41,42). To avoid overstating hypertension in SB, we used 50th percentile for age in lieu of actual height percentile for subjects with SB to determine z-scores for systolic blood pressure (43). Fasting serum samples were analyzed for glucose, insulin, TG, total cholesterol, HDL, and LDL concentrations.
Definitions of Metabolic Syndrome
Although several major studies have been conducted to assess the prevalence of metabolic syndrome in pediatric populations (44–46), there is no consensus for the cutoff values used to classify metabolic syndrome. Nevertheless, most studies appear to agree with classifying adolescents as having metabolic syndrome if they have ≥3 of the following criteria: obesity, insulin resistance/impaired glucose tolerance, high blood pressure, high TG, and low HDL. The values for obesity include a BMI z-score of ≥2.0 (97th percentile) adjusted for age and sex (45), 90th percentile BMI for age and sex, (46), total body fat mass as determined by DXA ≥30% in males and ≥35% in females (47), or a waist circumference of >90th percentile (13). Previously proposed criteria for obesity in adolescents with SB are 20% total body fat in males and 25% total body fat in females (48) and +2 root mean square of the residual from the regression of skinfold thickness and hydrostatic weighing (30). Insulin resistance has been defined 3 ways; impaired glucose tolerance based on a 2-hour postprandial blood glucose between 140 and 200 mg/dL (45), fasting blood glucose ≥110 mg/dL (13,44,46), and fasting insulin ≥75th percentile (49). Blood pressure has been indicated as high with either systolic or diastolic blood pressure >90th percentile for age, sex, and height (13,44,46), or with >95th percentile for age and sex (45). Triglycerides were considered high with 75th, (49) 90th (13,46), and 95th percentiles (44,45). Low HDL has been indicated with <5th (45), 10th (13,44), and 25th percentiles (49).
Therefore, given the lack of consensus, which makes comparison between studies somewhat difficult, we established the following criteria to determine risk factors in this study, as shown in Table 1, that while unique to our study, were based on Adult Treatment Panel III Guidelines to metabolic syndrome adjusted for gender, age, and puberty (44): abdominal adiposity for obesity categories were represented as percent trunk fat ≥30.0% for males and ≥35.0% for females (47); blood pressure ≥95th percentile for height, age, and gender; dyslipide-mia with HDL concentrations <45 mg/dL for males and <50 mg/dL for females and/or triglyceride levels ≥100 mg/dL; insulin resistance or impaired glucose tolerance represented as ≥1 of the following: fasting serum glucose 100 to 125 mg/dL, fasting serum insulin concentration ≥20 μU/mL, or the homeostasis model assessment of insulin resistance (HOMA-IR) ≥4.0 (8,50–52).
Table 1.
Cutoff Criteria Used to Classify Metabolic Syndrome Risk Factors*
We recognize that percent trunk fat is not the typical criterion that is used to measure obesity in the pediatric population; however, we believe that percent fat as measured by DXA has advantages compared with other indices of adiposity such as BMI and waist circumference in the SCI and SB populations because their body habitus has been shown to be significantly different from the normative population (29,36,37,39,53–56). One limitation with using DXA measured percent trunk fat is that it does not differentiate between visceral and subcutaneous adiposity; however, Svendsen et al found a correlation between DXA trunk fat percent and CT abdominal fat (r = 0.9) (57). Therefore, we studied the association between percent trunk fat determined by DXA with both waist circumference and BMI.
Biochemical Analysis
Serum glucose was measured using the Dade Behring Glucose Oxidase method, and serum insulin was measured by chemiluminescence with a Bayer reagent (Tarry-town, NY). HOMA-IR was calculated using the formula of Matthews et al (58) HOMA-IR = [(fasting plasma insulin concentration (mU/mL) × fasting plasma glucose (mmol/L)]/22.5. Serum HDL, total cholesterol, and TG were measured on a Beckman Coulter Synchron LX. LDL was calculated according to the Friedewald Formula.
Statistical Analysis of Data
A 3-factor ANOVA was used to assess whether diagnosis (CTRL, SCI, SB), obesity (nonobese <30.0% trunk fat in males and <35.0% in females, obese ≥30.0% trunk fat in males and ≥35.0% in females), or gender significantly affected each of the components that comprise metabolic syndrome. Nonsignificant interactions were removed in a stepwise hierarchical fashion. HOMA-IR and insulin were log transformed to conform to the normal distribution. Comparisons of differences between groups were made with post hoc tests using Tukey corrections. ANOVA and regression analysis were done with SPSS version 11.5 (SPSS, Chicago, IL). Chi-square analysis was used to determine frequency of all nonparametric measures including gender and age using Systat version 10 (SPSS, Chicago, IL).
RESULTS
Anthropometrics
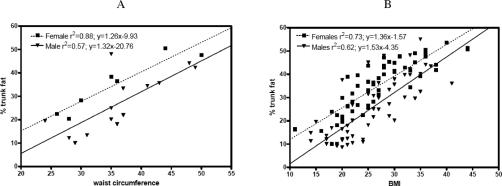
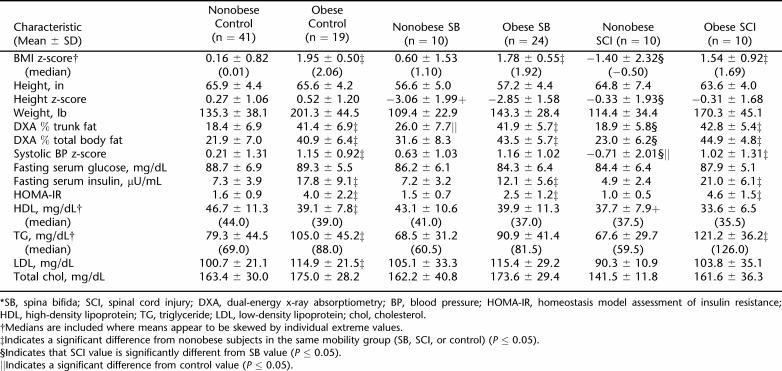
The demographic and metabolic characteristics of the obese and nonobese CTRL, SB, and SCI groups are shown in Tables 2 and 3. The 3 groups were not significantly different with respect to age (P = 0.548) and gender (P = 0.643). There was a significant difference in body weight between SB and CTRL, with SB weighing 14.6% less than CTRL (P < 0.001), but no significant difference between SCI and CTRL or between SB and SCI was observed. As shown in Figure 1A and B, there was a high degree of correlation between percent of trunk fat as determined by DXA with both waist circumference (r2 = 0.57 for males; r2 = 0.88 for females) and BMI (r2 = 0.62 for males; r2 = 0.73 for females). Percent total body fat and percent trunk fat as determined by DXA was significantly different between each group, with SB averaging 6.3% more trunk fat than SCI and 11.5% more trunk fat than CTRL (among all 3 groups, P < 0.001; and between SB and SCI and between SB and CTRL, P = 0.004). Obese SCI had been injured almost twice as long as nonobese SCI subjects (P < 0.001). There was a gender effect, with 10.4% higher total fat in female than in male subjects (P < 0.001), and an interaction between diagnosis and obesity (P =0.018). Females had 10.1% more trunk fat percentage than male subjects (P < 0.001). Height z-scores for SB were 3.3 lower than those of control subjects (2.9 ± 0.3 vs 0.4 ± 0.1; P < 0.001) and 2.6 lower than for SCI (2.9 ± 0.3 vs 0.3 ± 0.4; P < 0.001). Consequently, BMI z-scores were 0.7 higher in SB than CTRL and 1.36 higher in SB than SCI (1.4 ± 0.2 vs 0.7 ± 0.1; P < 0.001 and 1.4 ± 0.2 vs 0.1 ± 0.5; P < 0.001, respectively).
Table 2.
Demographics by Mobility Groups*
Table 3.
Metabolic Components by Mobility Groups*
Figure 1. Relationship of waist circumference to DXA % trunk fat (A). Male r2 = 0.57 (P = 0.0018); y = 1.32× − 20.76. Female r2 = 0.88 (P = 0.0017); y = 1.26× − 9.93. Relationship of BMI to DXA % trunk fat (B). Male r2 = 0.57 (P < 0.0001); y = 1.32× − 20.76. Female r2 = 0.88 (P < 0.0001); y = 1.26× − 9.93.
Blood Pressure
As shown in Table 3, there was no diagnosis effect for systolic blood pressure z-scores; however, there was a significant obesity effect and no diagnosis–obesity interaction effect. The systolic blood pressure z-scores were significantly higher in obese groups as compared to nonobese groups (overall 1.1 ± 0.2 vs 0.0 ± 0.2; P < 0.001) and between SCI and SB when comparing nonobese subjects only (0.7 ± 0.6 vs 0.6 ± 0.4; P = 0.0275).
Lipids
There were no significant differences in serum LDL concentrations between groups; however, LDL was 13.3 mg/dL higher in obese subjects than in nonobese subjects (113.0 ± 3.8 mg/dL vs 99.7 ± 2.9 mg/dL; P = 0.006). Similarly, while there were no differences in serum triglyceride concentrations between SB, SCI, and control groups (P = 0.231), triglyceride levels averaged 26.2 mg/dL higher in obese than in nonobese subjects (101.7 ± 5.9 mg/dL vs 75.5 ± 5.2 mg/dL; P = 0.001). Fasting TG was elevated in 26.5% of SB, 45.0% of SCI, and 29.3% of control subjects. Serum HDL concentrations were 8.5 mg/dL lower in SCI than in control subjects (P = 0.003), 6.2 mg/dL lower in obese groups (38.4 ± 1.3 mg/dL vs 44.6 ± 1.4 mg/dL; P =0.001), and 5.1 mg/dL lower in males than in females (39.0 ± 1.2 mg/dL vs 44.1 ± 1.6 mg/dL; P = 0.002). Although mean fasting serum HDL was lower in SB than in controls, as shown in Table 3, the difference was not statistically significant (P = 0.239); and the median values for HDL in SB and SCI were similar. Fasting HDL concentrations were lower than the cutoff in 73.5% of SB, 90.0% of SCI, and 67.2% of control subjects. There were no differences in total serum cholesterol between diagnosis groups or based on obesity; however, fasting serum total cholesterol concentrations in male subjects averaged 17.9 mg/dL lower than in female subjects (156.0 ± 3.3 mg/dL vs 174.0 ± 4.5 mg/dL; P = 0.002).
Serum Glucose and Insulin Resistance
Overall, differences in fasting serum glucose concentrations between SB and control groups were statistically significant (P = 0.012), although only 1 subject (obese control) had glucose intolerance. Fasting serum glucose was significantly lower in SB vs controls (P = 0.011), but fasting serum glucose was not significantly lower in SCI subjects versus controls (P = 0.222). Values for fasting serum insulin concentrations and HOMA-IR were more than doubled in obese subjects compared to nonobese subjects (15.8 ± 1.1 μU/mL vs 6.9 ± 0.5μU/mL for insulin, P < 0.001; and 3.4 ± 0.3 vs 1.5 ± 0.1 for HOMA-IR, P < 0.001). There was an interaction of diagnosis and obesity for insulin (P = 0.003) and for HOMA-IR (P = 0.004). Fasting insulin was elevated in 5.9% of SB, 30.0% of SCI, and 10.3% of control subjects; and 8.8% of SB, 30.0% of SCI, and 17.2% of control subjects had high HOMA-IR. Insulin and HOMA-IR correlated with systolic blood pressure (r = 0.227; P = 0.006 and r = 0.274; P = 0.006, respectively). HOMA-IR had a high correlation with trunk fat percentage (r = 0.614; P <0.001).
Metabolic Syndrome
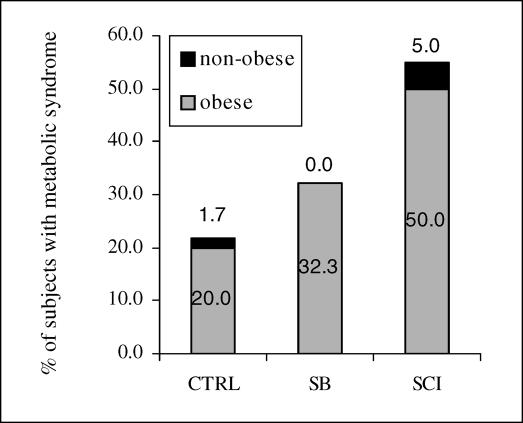
In our clinic population, 32% of SB subjects and 55.0% of SCI subjects met the criteria established for metabolic syndrome (≥3 risk factors/components) as shown in Figure 2. Only 5.9% of SB and 5.0% of SCI subjects had no components of metabolic syndrome, whereas 32.4% of SB and 35.0% of SCI subjects had 1 risk factor and 61.8% of SB and 60.0% of SCI subjects had ≥2 risk factors of metabolic syndrome. With the exception of only 2 subjects, those who had only 1 risk factor were not obese. All 5 indicators of metabolic syndrome were significantly affected by obesity, regardless of diagnostic group. As shown in Table 6, 100% of the obese SCI subjects, 45.8% of the obese SB subjects, and 63.2% of the obese CTRL subjects met the criteria for metabolic syndrome. A 3 × 2 contingency table revealed that there was a strong association between diagnostic group (SCI, SB, and CTRL) and presence of metabolic syndrome (χ2 = 0.012). The Fisher exact test demonstrated that the obese SCI group had a significantly higher percentage of subjects with metabolic syndrome than did the obese SB group (P < 0.005) and approached significance when compared to the CTRL group (P = 0.063). In contrast, there was no significant association between the presence of metabolic syndrome and diagnostic group in the nonobese subjects (P = 0.396). Only 10% of the nonobese SCI subjects, 0% of the nonobese SB subjects, and 2.4% of the nonobese control subjects met the criteria for metabolic syndrome. The most common 3 risk factors observed in this study were increased trunk fat, decreased fasting serum HDL concentration, and increased fasting serum triglyceride concentration, as shown in Table 5.
Figure 2. Prevalence of metabolic syndrome by group.
Table 6.
Percent With Increased Risk Factors by Group*
Table 5.
Percent of Individuals With Metabolic Syndrome Risk Factors by Group*
DISCUSSION
Our results represent the first known evidence that adolescents with SCI or SB and obesity have an increased prevalence of components of the metabolic syndrome, similar to what has been reported in adults with spinal cord injury (59,60) and able-bodied children with obesity (10,45,47,61–65). This finding is significant given that the occurrence of ≥3 components of the metabolic syndrome, documented in our study in many obese adolescents with spinal cord dysfunction, is associated with a threefold increased risk of developing cardiovascular disease and fivefold higher risk of developing diabetes, in addition to other comorbidities of obesity/diabetes such as diabetic neuropathy and nephropathy, polycystic ovary syndrome, sleep apnea, hepatic steatosis, gallstones, and certain types of cancer at an earlier age (11,66). Even though the sample sizes are small, the data from this study suggest that obese individuals with SCI have a significantly higher risk of metabolic syndrome than either obese SB subjects or obese able-bodied subjects do. The increased association of metabolic syndrome in obese SCI subjects as compared to obese SB subjects may be due to metabolic adaptation in utero and/or during development for subjects with SB. Although obese SCI subjects had a greater prevalence of metabolic syndrome than obese SB subjects, the importance of obesity in SB should not be overlooked since there is an increased prevalence of obesity among children with thoracic and upper lumbar spinal cord dysfunction due to SB and SCI associated with lower limb paralysis and loss of lean tissue in the lower extremities (28,29,31,33,36,37,39,53–56,67), reduced resting energy expenditure (28,67), increased sedentary existence (68), and reduced fitness levels (69).
Obesity is the primary contributing factor for both able-bodied adolescents and mobility-impaired adolescents with SCI and SB to develop additional risk factors in the constellation referred to as the metabolic syndrome; however, determining the most appropriate assessment of obesity is problematic in subjects with SCI and SB. The loss of lower limb muscle mass and segmental growth retardation of limbs due to paralysis, increase in total body and trunk fat, increasing regional and total body adiposity, and decreasing lean tissue with age, as well as the occurrence of progressive skeletal deformities in the lower limb and trunk, skew BMI calculations, resulting in overestimation of BMI in nonobese SB and underestimation in SCI subjects (29,70). Since percent trunk fat by DXA correlated well against waist circumference as shown in Figure 1A, we used the percent trunk fat as an indicator of central adiposity for all subjects regardless of mobility status. As a result, 74% of SB subjects and 50% of SCI subjects were classified as overweight based on the percentage of trunk fat when determining whether they met the criteria for obesity, as opposed to only 52.9% and 25.0%, respectively, based on BMI z-score. Seventy-five percent of SB females were obese compared to 66.7% of SB males. These results concur with those by Shurtleff and others that in adolescence, female subjects with SB have increased fat mass compared to male subjects with SB (36,37,71). Total body fat is approximately 18% in ambulatory SB in compared to ≥31% in nonambulatory SB (29,31,56), and 58% of SB are obese (30). The finding that paraplegic SCI subjects with greater amounts of total body fat had longer duration of injury agrees with those of Manns et al (59). The NHANES III disability survey reported 30% increased prevalence of obesity in participants with a disability (30% vs 23%) (72,73).
One hundred percent of obese SCI subjects and 45.8% of obese SB subjects in our study met the criteria for metabolic syndrome (≥3 risk factors/components), as compared to the reported 30% occurrence of metabolic syndrome in overweight and obese adolescents from the NHANES III data (13,44,45,72,73). Very low proportions of nonobese subjects studied had all 5 components of the metabolic syndrome (approximately 5% of SCI, 0% of SB, and 3% of control subjects), comparable to 0% of able-bodied children reported in NHANES III (13). SCI, SB, or able-bodied adolescents who do not become obese have a low likelihood of exhibiting components of the metabolic syndrome. Regardless of the presence or absence of spinal cord impairment, ≥10% of nonobese subjects studied had ≥3 components of the metabolic syndrome. It would be worth exploring in the future whether complete vs incomplete injury to the spinal cord as opposed to location of the injury itself accounts for the different rates of metabolic syndrome in patients with spinal cord dysfunction.
Risks associated with increased systolic blood pressure in obese adolescent subjects have been previously documented (74–77). Therefore, increased systolic blood pressure observed in our obese subjects is of concern because there is both increased incidence of hypertension and an increase in intimal–medial thickness in the carotid arteries (77). Furthermore, the Muscatine Study data, which tracked 754 children and adolescents (mean age 15 y; range 8–18 y) into adulthood (mean age 44 y; range 40–49 y) revealed increased systolic blood pressure in adolescence persisted into adulthood, especially when they remained obese as adults (76).
Studies of lipid profiles in disabled populations have primarily been conducted in adults. Obesity with dyslipidemia in 45% of adult SCI subjects has been previously identified (78). Although SB adolescents in this study had decreased levels of HDL and increased levels of LDL and total cholesterol compared to controls, the differences were not statistically significant. Rendeli et al also reported no significant differences in lipid profiles between SB children and adolescents (ages 1–16 y) and able-bodied controls (79). The trend towards decreased HDL and increased TG observed in our spinal cord dysfunction groups agrees with the results found in adult neuromuscular disease patients by Aitkens and colleagues (80). A higher proportion of their disabled group had decreased HDL and increased systolic blood pressure (80). They identified decreased HDL, increased BMI, and increased TG as the top 3 risk factors in both their disabled population and able-bodied control group. This corresponds with the top 3 risk factors observed in our study—increased percent trunk fat, decreased HDL, and elevated TG—and is also consistent with the study of overweight adolescents by de Ferranti et al (44). We observed 74.0% SB, 90.0% SCI, and 65.0% of CTRL to have decreased HDL and increased systolic blood pressure. Values for fasting serum HDL concentrations were lower in SCI than controls. Bauman et al observed decreased HDL concentrations in SCI compared with control subjects, with significant differences reported in males, whites, and Hispanics, but not in females or African Americans (81–84). SCI subjects in our study had HDL values <35 mg/dL at a rate of 35%, which corresponded to 24 to 40% of SCI in studies by Bauman et al. Forty-five percent of subjects with SCI in our study had HDL ≤45 mg/dL. These results are similar to those of Dopler-Nelson et al, who found that 49% of adults with SCI had significantly increased TG and 30% had decreased HDL (85). Since decreased HDL is an independent risk factor for increased myocardial infarction in adulthood and appears to be due to immobilization (82,86,87), these results further suggest that adolescents with spinal cord dysfunction who develop components of the metabolic syndrome will be at increased risk for cardiovascular disease in adulthood.
Although normal fasting blood glucose was observed in 99% of subjects in this study, they may still be at risk for developing diabetes (10). SB, SCI, and able-bodied subjects with documented type 1 or type 2 diabetes were excluded from our study. It was perhaps surprising that SB subjects had lower rates of insulin resistance than SCI subjects based on HOMA-IR, given that SB are disabled from birth and the percent trunk fat and percent total body fat are similar in obese SB and obese SCI. It is possible that the differences observed between SB and SCI may be due to a higher proportion of visceral fat in SCI subjects (88).
HOMA-IR level was not significantly different among SB, SCI, and control subjects. These interpretations are based on insulin values from 1 fasting time point and may indicate a different outcome had they been based on a 2-hour postprandial glucose load; however, HOMA-IR has been indicated as a valid measure of impaired glucose tolerance in lieu of a glucose challenge (8,89). Insulin resistance in spinal cord dysfunction appears to be directly related to denervation of skeletal muscle (90). There were no SB or SCI subjects with glucose intolerance; however, 8.8% of SB, 30.0% of SCI, and 16.7% of controls had hyperinsulinemia and/or insulin resistance based on fasting insulin concentrations and/or HOMA-IR values, even though known diabetes was an exclusion criterion. This is approximately half the rate reported by Jones et al in their study of 20 SCI male adults (78). The relationship of HOMA-IR to BMI is inconsistent with that observed by Bacha et al (r2 = 0.308 in our study, compared to r2 = 0.73) (91). Associations between HOMA-IR and BMI z-score and between HOMA-IR and systolic blood pressure z-score were also reported by Arnlov et al (92). They found that insulin resistance in younger adults with normal BMI and blood pressure was predictive of future hypertension. All of the SCI subjects in this study with insulin resistance were obese with dyslipidemia. Obesity in adolescence tracks into adulthood and consequently increases the risk of developing metabolic syndrome. Vanhala et al showed that of 30 adults with metabolic syndrome, 93% were obese adults and 70% had been obese as children (21). Fifty percent to 80% of overweight children will remain overweight as adults (93,94). Children with BMI ≥75th percentile followed for approximately 12 years in the Bogalusa Heart Study had 11.7 times the rate of having metabolic syndrome as adults (87). The number of risk factors in youth is predictive of decreased carotid artery elasticity in adulthood, with systolic blood pressure as an independent predictor (17). This may account for the increased incidence of cardiovascular disease as a leading cause of mortality in SCI at a younger age than in the general population (95–98). Most alarming is the overall pattern of the increased risk in adolescence for developing complications due to a more sedentary lifestyle and increased prevalence of obesity at a younger age than in previous generations.
CONCLUSIONS
The results of this study document that subjects with SCI or SB have a very high risk of having components of the metabolic syndrome, and this increased risk is primarily due to their increased prevalence of obesity. Obese able-bodied subjects, obese SB subjects, and obese subjects with SCI had high rates of abdominal adiposity as assessed by percent trunk fat and body fat percentage, and an increased number of components of the metabolic syndrome, mainly low serum HDL concentrations, high TG levels, and insulin resistance compared with nonobese control, SB, and SCI subjects. In our sample, the obese SCI subjects had a higher prevalence of metabolic syndrome than the obese SB and obese able-bodied subjects did. Prevention of obesity in children with spinal cord dysfunction will likely be critical to the avoidance of metabolic syndrome components and the promotion of optimal health. Ideally, this should begin in infancy in SB and very soon after the occurrence of a traumatic SCI. This early emphasis on health promotion for children and adolescents with spinal cord dysfunction will require nutrition education, modified caloric intake to achieve a more optimal balance of energy intake and energy expenditure, and exercise including strengthening, aerobic exercise, and community physical activity and recreation participation (69). Prevention of obesity and management of metabolic syndrome components among obese patients should be a high priority in any rehabilitation program for children and adolescents with spinal cord dysfunction.
Table 4.
Metabolic Components by Mobility Groups and Obesity*
Footnotes
Supported in part by Shriners Hospitals for Children Project number 8600: Exercise and Dietary Intervention in Obese Children with Paraparesis due to Spinal Cord Dysfunction; National Institute of Disability and Rehabilitation Research Grant #H133B031118; Rehabilitation Research and Training Center in Neuromuscular Diseases: Enhancing Health, Function, and Quality of Life; National Institute of Child Health and Human Development Grant # RO1 HD35714: Child Mobility: Role of Strength, Body Fat & Energy Cost.
REFERENCES
- Behn A, Ur E. The obesity epidemic and its cardiovascular consequences. Curr Opinion Cardiol. 2006;21:353–360. doi: 10.1097/01.hco.0000231406.84554.96. [DOI] [PubMed] [Google Scholar]
- Rigby NJ, Kumanyika S, James WP. Confronting the epidemic: the need for global solutions. J Public Health Policy. 2004;25:418–434. doi: 10.1057/palgrave.jphp.3190040. [DOI] [PubMed] [Google Scholar]
- Cheng TO. Obesity is a global challenge (Comment) Am J Med. 2006;119:E11. doi: 10.1016/j.amjmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Stubbs CO, Lee AJ. The obesity epidemic: both energy intake and physical activity contribute. Med J Austr. 2004;181:489–491. doi: 10.5694/j.1326-5377.2004.tb06406.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Pagliassotti M, Peters J. Genetic Determinants of Obesity. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Li SM, Zhou X-H. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- Paulsen EP, Richende L, Ginsberg-Fellner F. Plasma glucose free fatty acids and immunoreactive insulin in 66 obese children—studies in reference to a family history of diabetes mellitus. Diabetes. 1968;17:261–269. doi: 10.2337/diab.17.5.261. [DOI] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. Br Med J. 2006;332:878–881. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- Najarian RM, Sullivan LM, Wilson PF, D'Agostino RB, Kannel WB, Wolf PA. Impact of metabolic syndrome compared to diabetes as a risk factor for stroke: Framing-ham Offspring study. Stroke. 2006;166:106–111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51:204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JSA, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood—the cardiovascular risk in young Finns study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Maison P, Byrne CD, Hales CN, Day NE, Wareham NJ. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care. 2001;24:1758–1763. doi: 10.2337/diacare.24.10.1758. [DOI] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- Vanhala M, Vanhala P, Kumpusalo E, Halonen P, Takala J. Relation between obesity from childhood to adulthood and the metabolic syndrome: population based study. Br Med J. 1998;317:319. doi: 10.1136/bmj.317.7154.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Bandini LG, Schoeller DA, Fukagawa NK, Wykes LJ, Dietz WH. Body-composition and energy-expenditure in adolescents with cerebral-palsy or myelodysplasia. Pediatr Res. 1991;29:70–77. doi: 10.1203/00006450-199101000-00014. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77:371–378. doi: 10.1093/ajcn/77.2.371. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev. 2004;41:1–8. doi: 10.1682/jrrd.2004.01.0001. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Body composition changes and anabolic hormone considerations with advancing age and in persons with spinal cord injury. Wounds Compend Clin Res Pract. 2001;13:22D–31D. [Google Scholar]
- Bauman W, Spungen A. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11:109–140. [PubMed] [Google Scholar]
- Liusuwan A, Widman L, Abresch T, McDonald CM. Altered body composition affects resting energy expenditure and interpretation of body mass index in children with spinal cord injury. J Spinal Cord Med. 2004;27:S24–S28. doi: 10.1080/10790268.2004.11753781. [DOI] [PubMed] [Google Scholar]
- Shepherd K, Roberts D, Golding S, Thomas BJ, Shepherd RW. Body composition in myelomeningocele. Am J Clin Nutr. 1991;53:1–6. doi: 10.1093/ajcn/53.1.1. [DOI] [PubMed] [Google Scholar]
- Mita K, Akataki K, Itoh K, Ono Y, Ishida N, Oki T. Assessment of obesity of children with spina bifida. Dev Med Child Neurol. 1993;35:305–311. doi: 10.1111/j.1469-8749.1993.tb11642.x. [DOI] [PubMed] [Google Scholar]
- Roberts D, Shepherd RW, Shepherd K. Anthropometry and obesity in myelomeningocele. J Paediatr Child Health. 1991;27:83–90. doi: 10.1111/j.1440-1754.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Bonet Serra B, Cocho Gomez P, Quintanar Rioja A, Bueno Campana M, Espino Hernandez M. Metabolic alterations related to syndrome X and low vitamin E levels in obese children with acanthosis nigricans. Ann Pediatr (Barc) 2004;60:142–147. doi: 10.1016/s1695-4033(04)78235-1. [DOI] [PubMed] [Google Scholar]
- Olle MM, Pivarnik JM, Klish WJ, Morrow JR. Body-composition of sedentary and physically active spinal-cord injured individuals estimated from total-body electrical-conductivity. Arch Phys Med Rehabil. 1993;74:706–710. doi: 10.1016/0003-9993(93)90030-e. [DOI] [PubMed] [Google Scholar]
- Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury—a retrospective study. Spinal Cord. 2006;44:92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Protocol. http://www.cdc.gov/nchs/data/nhanes/nhanes_general_guidelines_june_04.pdf. Accessed May 20, 2005.
- Hayes-Allen MC. Obesity and short stature in children with myelomeningocele. Dev Med Child Neurol Suppl. 1972;27:59–64. doi: 10.1111/j.1469-8749.1972.tb09775.x. [DOI] [PubMed] [Google Scholar]
- Shurtleff D, Lamers J, Goiney T, et al. Are myelodysplastic children fat? Anthropometric measures: a preliminary report. Spina Bifida Ther. 1982;4:1–21. [Google Scholar]
- Yamane T, Yamashita T, Nakagawa T, et al. Habilitation problems of school-aged children with open myelomeningocele. Sogo Rehabil. 1990;18:183–188. [Google Scholar]
- Bandini LG, Schoeller DA, Fukagawa NK, Wykes L, Dietz WH. Body-composition and basal metabolic-rate (BMR) in children and adolescents with myelodysplasia. Am J Clin Nutr. 1988;47:776–776. [Google Scholar]
- McDonald CM, Abresch RT, Widman L, Styne DM, Warden N, Kilmer DD. Body composition and water compartment measurements in boys with Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2005;84:483–491. doi: 10.1097/01.phm.0000166880.91117.04. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Freedman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- National High Blood Pressure Education Program The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moran M, Salazar-Vazquez B, Violante R, Guerrero-Romero F. Metabolic syndrome among children and adolescents aged 10–18 years. Diabetes Care. 2004;27:2516–2517. doi: 10.2337/diacare.27.10.2516. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Moreno LA, Blay MG, et al. Body composition in adolescents: measurements and metabolic aspects. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S54–S58. doi: 10.1038/sj.ijo.0802805. [DOI] [PubMed] [Google Scholar]
- Huenemann R, Hampton M, Shapiro L, Behnke A. “Adolescents” food practices associated with obesity. Federal Proceedings. 1966;25:4–10. [PubMed] [Google Scholar]
- Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services—recommendations from an expert committee. Am J Clin Nutr. 1994;59:307–316. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. Br Med J. 2005;330:1363–1364. doi: 10.1136/bmj.38429.473310.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascaso JF, Romero P, Real JT, Priego A, Valdecabres C, Carmena R. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non-diabetic population. Medicina Clinica. 2001;117:530–533. doi: 10.1016/s0025-7753(01)72168-9. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders—the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Healthy body mass index values often underestimate body fat in men with spinal cord injury. Arch Phys Med Rehabil. 2003;84:1068–1071. doi: 10.1016/s0003-9993(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Bauman W. Carbohydrate and lipid metabolism in individuals after spinal cord injury. Top Spinal Cord Inj Rehabil. 1997;2:1–22. [Google Scholar]
- Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- Asher M, Olson J. Factors affecting the ambulatory status of patients with spina-bifida cystica. J Bone Joint Surg Am. 1983;65:350–356. [PubMed] [Google Scholar]
- Svendsen OL, Haarbo J, Hassager C, Christiansen C. Accuracy of measurements of body-composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr. 1993;57:605–608. doi: 10.1093/ajcn/57.5.605. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment—insulin resistance and beta-cell function from fasting plasma-glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005;86:1176–1181. doi: 10.1016/j.apmr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37:601–616. doi: 10.1038/sj.sc.3100911. [DOI] [PubMed] [Google Scholar]
- Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–14. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int J Obes Relat Metab Disord. 2004;28:833–841. doi: 10.1038/sj.ijo.0802694. [DOI] [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents—findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Practice Res Clin Endocrinol Metab. 2005;19:405–419. doi: 10.1016/j.beem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome—report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Liusuwan R, Widman L, Abresch RT, Styne DM, McDonald CM. Body composition and resting energy expenditure in pediatrics aged 11 to 21 years with spinal cord dysfunction compared to able-bodied controls: comparisons and relationships among the groups. J Spinal Cord Med. 2007;30(Suppl 1):S108–S114. doi: 10.1080/10790268.2007.11754613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Widman L, Walsh S, Page P, Abresch R. Community heart rate monitoring in disabled populations as a measure of physical activity. J Spinal Cord Med. 2004;27:S118–S119. [Google Scholar]
- Widman L, Abresch R, Styne D, McDonald C. Aerobic fitness and upper extremity strength in patients aged 11 to 21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med. 2007;30(Suppl 1):S91–S99. doi: 10.1080/10790268.2007.11754611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Abresch-Meyer A, Dopler Nelson M, Widman L. Body mass index and body composition measured by dual-energy x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med. 2007;30(Suppl 1):S100–S107. doi: 10.1080/10790268.2007.11754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden PW, Davenport SLH, Campbell MM. Adolescents with myelodysplasia—impact of physical-disability on emotional maturation. Pediatrics. 1979;64:53–59. [PubMed] [Google Scholar]
- Centers for Disease Control State-specific prevalence of obesity among adults with disabilities—eight states and the District of Columbia, 1998–1999. Morb Mortal Wkly Rep. 2002;51:805–808. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm513691.htm. Accessed December 11, 2005. [PubMed] [Google Scholar]
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion Healthy People 2010. http://wonder.cdc.gov/data2010. Accessed December 11, 2005. [PubMed]
- Israeli E, Schochat T, Korzets ZE, Tekes-Manova D, Bernheim J, Golan E. Prehypertension and obesity in adolescents: a population study. Am J Hypertens. 2006;19:708–712. doi: 10.1016/j.amjhyper.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Kim HM, Park J, Kim H-S, Kim DH, Park SH. Obesity and cardiovascular risk factors in Korean children and adolescents aged 10–18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am J Epidemiol. 2006;164:787–793. doi: 10.1093/aje/kwj251. [DOI] [PubMed] [Google Scholar]
- Burns TL, Letuchy EM, Lauer RM. Childhood blood pressure and change in body mass index from childhood to adulthood predict hypertension in the Muscatine Study adult cohort. Circulation. 2006;113:E303–E303. [Google Scholar]
- Stabouli S, Kotsis V, Papamichael C, Constantopoulos A, Zakopoulos N. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal-medial thickness. J Pediatr. 2005;147:651–656. doi: 10.1016/j.jpeds.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Factor analysis of the metabolic syndrome in spinal cord-injured men. Metab Clin Exp. 2004;53:1372–1377. doi: 10.1016/j.metabol.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Rendeli C, Castorina M, Ausili E, et al. Risk factors for atherogenesis in children with spina bifida. Childs Nerv Syst. 2004;20:392–396. doi: 10.1007/s00381-004-0912-8. [DOI] [PubMed] [Google Scholar]
- Aitkens S, Kilmer DD, Wright NC, McCrory MA. Metabolic syndrome in neuromuscular disease. Arch Phys Med Rehabil. 2005;86:1030–1036. doi: 10.1016/j.apmr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observations from a diverse cohort. Spinal Cord. 1999;37:485–493. doi: 10.1038/sj.sc.3100862. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high-density-lipoprotein cholesterol levels in veterans with spinal-cord injury. Paraplegia. 1992;30:697–703. doi: 10.1038/sc.1992.136. [DOI] [PubMed] [Google Scholar]
- Brenes G, Dearwater S, Shapera R, Laporte RE, Collins E. High-density-lipoprotein cholesterol concentrations in physically active and sedentary spinal-cord injured patients. Arch Phys Med Rehabil. 1986;67:445–450. [PubMed] [Google Scholar]
- Hooker SP, Wells CL. Effects of low-intensity and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc. 1989;21:18–22. doi: 10.1249/00005768-198902000-00004. [DOI] [PubMed] [Google Scholar]
- Dopler-Nelson M, Burri BJ. Dietary intake in the spinal cord injured patient. Am J Clin Nutr. 2002;75:438S–439S. [Google Scholar]
- von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- Sprecher DL, Watkins TR, Behar S, Brown WV, Rubins HB, Schaefer EJ. Importance of high-density lipoprotein cholesterol and triglyceride levels in coronary heart disease. Am J Cardiol. 2003;91:575–580. doi: 10.1016/s0002-9149(02)03309-x. [DOI] [PubMed] [Google Scholar]
- Taksali S, Dziura J, Goodman R, Burgert T, Papademtris X, Caprio S. High Prevalence of metabolic syndrome in obese youth with increased visceral fat. American Diabetes Association Conference [Abstract] 2006. p. 1769-P.
- Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- Buse MG, Buse J. Glucose uptake and response to insulin of the isolated rat diaphragm—the effect of denervation. Diabetes. 1959;8:218–225. doi: 10.2337/diab.8.3.218. [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth— relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- Arnlov J, Pencina MJ, Nam B-H, et al. Relations of insulin sensitivity to longitudinal blood pressure tracking: variations with baseline age, body mass index, and blood pressure. Circulation. 2005;112:1719–1727. doi: 10.1161/CIRCULATIONAHA.105.535039. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- Garn SM. Continuities and changes in fatness from infancy through adulthood. Curr Probl Pediatr. 1985;15:1–47. doi: 10.1016/0045-9380(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Capoor J, Stein AB. Aging with spinal cord injury. Phys Med Rehabil Clin N Am. 2005;16:129–161. doi: 10.1016/j.pmr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Gerhart KA, Bergstrom E, Charlifue SW, Menter RR, Whiteneck GG. Long-term spinal-cord injury—functional changes over time. Arch Phys Med Rehabil. 1993;74:1030–1034. doi: 10.1016/0003-9993(93)90057-h. [DOI] [PubMed] [Google Scholar]