Abstract
Background/Objectives:
To assess patient and provider behaviors regarding influenza vaccination, diagnosis, and testing strategies and the availability of influenza vaccine during the 2004–2005 nationwide influenza vaccine shortage.
Design/Methods:
Multisite, anonymous, cross-sectional surveys of patients and providers and qualitative interviews after the 2004–2005 influenza season.
Setting:
Department of Veterans Affairs (VA) health care facilities with spinal cord injury centers or clinics.
Participants:
Stratified random sample of 3,958 veterans with spinal cord injuries and disorders (SCI&D; 31 % response rate), 177 providers who treat persons with SCI&D, and 17 key informants.
Results:
Most patient respondents (96.1%) reported awareness of a vaccine shortage (n = 938). When asked whether the shortage affected their ability to get the vaccine, 64.8% said they had no problem, whereas 12.1% reported an inability to get the vaccine. The vaccination rate was 71.8%; most veterans received the vaccine early (October–November) at the VA, and vaccination rates increased with age (P < 0.0001). Although vaccine shortages were reported by 47.5% of provider survey respondents (n = 177), most reported that the vaccine shortage did not affect availability of vaccine for patients with SCI&D. Few clinicians conducted diagnostic tests for influenza more often than in past years (4.9%). Although providers reported shortages at 12 centers (n = 23), patients with SCI&D had priority at 11 of 12 centers.
Conclusions:
Most patients were aware of the vaccine shortage, and the vaccination rate remained high and comparable with previous years. VA providers and facilities targeted SCI&D as a high-risk group and prioritized use of the limited vaccine supply for them.
Keywords: Influenza, Vaccination, Health care workers, Spinal cord injuries, Prevention
INTRODUCTION
Just before the 2004–2005 influenza vaccination season, the license of 1 of the 2 national suppliers of influenza vaccine to the United States was suspended. This resulted in a widespread vaccine shortage during the 2004–2005 influenza vaccination season, which received considerable national and local media coverage. Interim recommendations were issued by the Centers for Disease Control and Prevention (CDC) to address the shortage (1).
Three recent reports have been published on the effects of the vaccine shortage in the United States on vaccination rates and the public's perceptions of the shortage. The Harvard School of Public Health, in collaboration with International Communications Research, conducted a national survey to assess the experiences with obtaining influenza vaccination among persons in the priority groups set by the CDC during the vaccine shortage (2,3. Among adult respondents who were at least 65 years of age, 49% attempted to get the vaccine; of those who tried to get the vaccine, 37% could not get it. In adults with chronic illnesses, 37% attempted to get the vaccine and only 46% received the vaccine. The most common reasons cited for not obtaining the vaccine among the priority groups were no vaccine was available when they went to get it (68%), it was hard to find a place to get the vaccine (50%), the times when the vaccine was available were inconvenient (24%), a health care provider did not place them in a high-risk priority group (15%), vaccine was expensive (4%), and health care provider said the vaccine was contraindicated (4%) (2,3).
A national survey of members of more than 250 managed care organizations focused on assessing vaccination rates in persons 50 to 64 years of age (4). The authors found that between 2003–2004 and 2004–2005, vaccination coverage for this population decreased from 52.4% to 28.1%, which is a 46% decrease that was found across all age groups. Self-reported health status was also related to vaccination coverage, with healthier respondents less likely to receive a vaccination. The vaccination rate for those reporting excellent health decreased by 51.6% from one season to the next, whereas the decline in those who reported poor health decreased by 23.5% (4). These study results suggest that the limited availability of vaccine, media coverage, and prioritization of vaccine had a considerable effect on vaccination rates. Respondents who were older or who reported poorer health status had smaller reductions in vaccination coverage, suggesting that efforts to target higher-risk members were moderately successful (4).
The most recent study on the influenza vaccine shortage from 2004–2005 focused on racial and ethnic disparities in influenza vaccination coverage among adults (5). The authors identified disparities in vaccination coverage between ethnic groups. Regression analysis showed that, by the end of January 2005, non-Hispanic blacks and Hispanics were less likely to have received the vaccine than non-Hispanic whites. Other factors associated with receiving the vaccine were being 75 years of age or older (compared with those 65–75 years), having a higher education level, having a chronic condition, being a health care worker, and living in a state where the ratio of supply of vaccine to the number of vaccine priority groups was high (5).
Persons with spinal cord injuries and disorders (SCI&D) are at increased risk of respiratory complications and death if they contract influenza because of their impaired respiratory function. Thus, the importance of obtaining vaccines for this group was paramount. The Department of Veterans Affairs (VA) received a sufficient amount of influenza vaccine to vaccinate all veterans at risk or who wanted the vaccine for the 2004–2005 influenza season, because the majority of vaccine was purchased from the supplier whose product was unaffected. However, at the time the national shortage was announced, there was considerable concern about adequate supplies in the VA and the general community. The VA SCI&D Strategic Healthcare Group (SHG) and the Spinal Cord Injury Quality Enhancement Research Initiative (SCI QUERI) had promoted ongoing education of patients and providers for the prior 5 years regarding the risks of influenza in persons with SCI&D, the need to be vaccinated (6–8), and diagnostic and treatment options (9). Furthermore, during the 2002–2003 vaccination season, the VA added SCI&D to their list of high-priority high-risk groups for influenza vaccination. The influenza vaccine shortage provided an opportunity to further these educational efforts through quality improvement strategies and to assess the effects of the shortage on patient vaccination behavior, health care provider behavior in providing the vaccine, and provider strategies for diagnostic testing and treatment of influenza in those with SCI&D.
The primary goal of this study was to assess patient and provider behaviors regarding vaccination in a high-risk group—veterans with SCI&D. Providers were surveyed to assess the availability of influenza vaccine at VA SCI centers. Secondary goals were to learn about provider testing and treatment strategies in relation to influenza during a national shortage in a high-risk population treated in the VA health care system.
METHODS
Study Design
This was a multisite, cross-sectional, observational study at 23 VA SCI centers, SCI outpatient clinics, and facilities with primary care teams. This study was approved by the Edward Hines Jr. VA Hospital and University of Washington (for the VA Puget Sound VA Healthcare System) Human Studies committees. In spring of 2005, anonymous surveys were distributed to veterans with SCI&D and health care providers (eg, physicians, physician assistants, nurse practitioners, nurses, therapists, and psychosocial workers) who care for veterans with SCI&D. Interviews were also conducted with key informants at VA SCI centers about the availability of influenza vaccine at the SCI center, whether veterans with SCI&D were a priority group for the vaccine, and activities to notify veterans and providers about influenza vaccine.
Patient Survey
Surveys for veterans with SCI&D were mailed using local registry lists maintained by each SCI center. The survey mailing list, updated from the returned mail from earlier surveys (8), contained 6,090 veterans with SCI&D. A random sample of veterans, stratified by SCI center, was drawn from the updated list for each SCI center for survey distribution. The sample represented 65% of the veterans from each center (total n = 3,958). The patient survey was mailed anonymously, so we were unable to determine differences between respondents and nonrespondents or determine an accurate response rate.
The current survey was 2 pages long and included questions on whether or not the respondent received an influenza vaccination in the past year, and if not, why and questions regarding awareness of the influenza vaccine shortage and how it affected their ability to get the vaccine. In addition, questions were included regarding self-reported health status and demographics (ie, age, sex, education, race, and level of injury).
Provider Survey
The provider survey was an internet-based survey set up on the SCI QUERI website. SCI&D provider e-mail distribution lists were used to send letters describing the purpose of the study and the website link to completing the survey. The e-mail lists included VA staff physicians, nurses and nurse managers, therapists (physical, occupational, and kinesiology), psychologists, social workers, and SCI center chiefs. It is estimated that approximately 600 providers were on these distribution lists, with some overlap in providers among the groups and some nonproviders on the lists (eg, researchers, advocacy group representatives). The survey was completed by respondents anonymously; thus, we were unable to directly compare respondents to nonrespondents of the survey or determine an accurate response rate. The characteristics of the survey respondents were compared with a previous survey on influenza vaccination practices among health care workers providing care to veterans with SCI&D (7). The current survey included questions about the influenza vaccine shortage for 2004–2005, use of diagnostic tests for influenza, and treatment strategies for influenza for veterans with SCI&D. Questions on self-reported vaccination status and demographic information were also posed.
Key Informant Interviews
Semistructured telephone interviews were conducted with nurse managers, head nurses, charge nurses, registered nurses, or SCI coordinators at 17 of 23 VA SCI centers. Interviews inquired about availability of the influenza vaccine and influenza vaccination activities and strategies used during the 2004–2005 influenza vaccination season.
Analysis
This was a descriptive study using frequencies to describe data. All bivariate analyses were compared using the χ2 test. Analyses were performed using Stata 8.0 (Stata Corporation, College Station, TX). For the key informant interview data, yes and no responses about the shortage of vaccine were tallied separately. For the question about availability of vaccine in SCI centers, descriptions were summarized.
RESULTS
Patient Survey
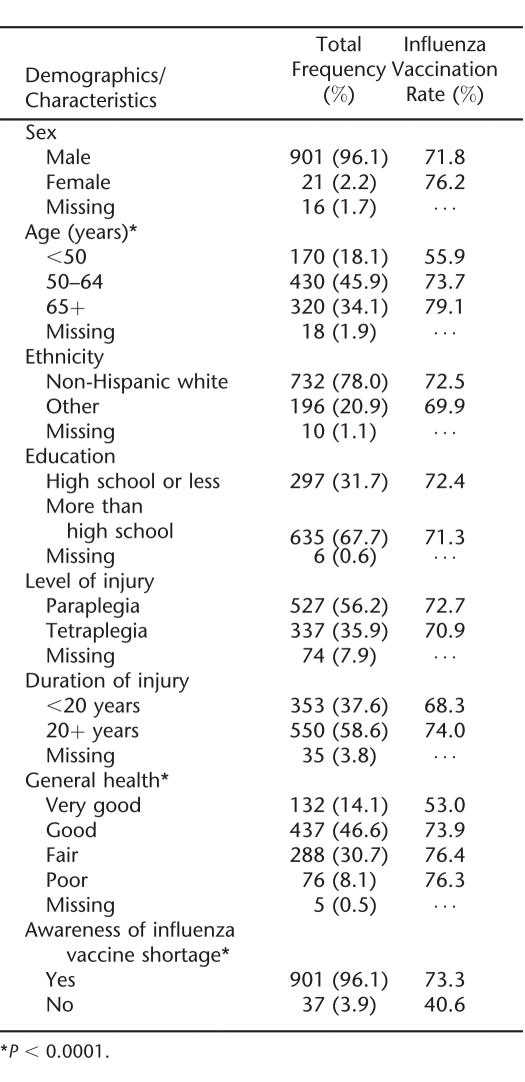
Of the 3,958 veterans mailed the survey, 31% (968) responded. Subjects who did not complete the main questions of the survey, which asked about receipt of an influenza vaccination or awareness of the impact of the shortage, were excluded from the study (3.1% of the sample). The analyses included 938 veterans. Most respondents were men, white, at least 50 years of age with paraplegia, and had been injured for 20 or more years. Almost two thirds of respondents had more than a high school education (Table 1).
Table 1.
Survey Respondents' Demographics, Characteristics, and Influenza Vaccination Rates (n = 938)
Most respondents (96.1%) reported that they were aware that there was a vaccine shortage during the 2004–2005 influenza season (Table 1). Among those aware of the shortage, more than two thirds (64.8%) said they had no problem getting the vaccine, whereas 16.2% said they chose not to get the vaccine, 12.1% said the shortage affected them and they had to get the vaccine later in the year, 3.6% said they chose not to get the vaccine so that others could get it, and 3.3% reported they were unable to get the vaccine.
More than 70% (71.8%) of respondents were vaccinated during the 2004–2005 season. Most received the vaccine in either October or November 2004 (81.4%), 10.7% received the vaccine in December 2004, and 7.9% received it from January through March 2005. Most respondents received the vaccine at the VA (81.9%). The vaccination rate was significantly higher in older age groups: 55.9% for those less than 50 years, 73.7% for the 50- to 64-year age group, and 79.1% for the 65 years and older age group (P < 0.0001; Table 1). Those reporting fair or poor general health were more likely to receive the vaccine than those reporting very good or good general health (P = 0.02). The vaccination rate was significantly higher in those who were aware of the vaccine shortage compared with those who were not (P < 0.0001). No other demographics or characteristics were significantly associated with receiving the vaccine.
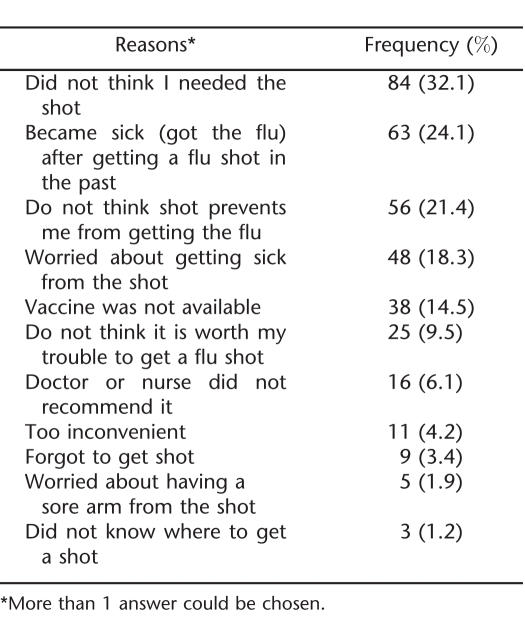
The most common reasons respondents reported for not receiving the vaccine included thinking they did not need the vaccine, reporting that they developed influenza from the vaccination in the past, or thinking that the vaccine does not prevent them from getting influenza (Table 2). Fifteen percent reported they did not get the vaccine because it was not available.
Table 2.
Reasons for Not Receiving a Flu Shot in Survey Respondents (n = 265)
Provider Survey
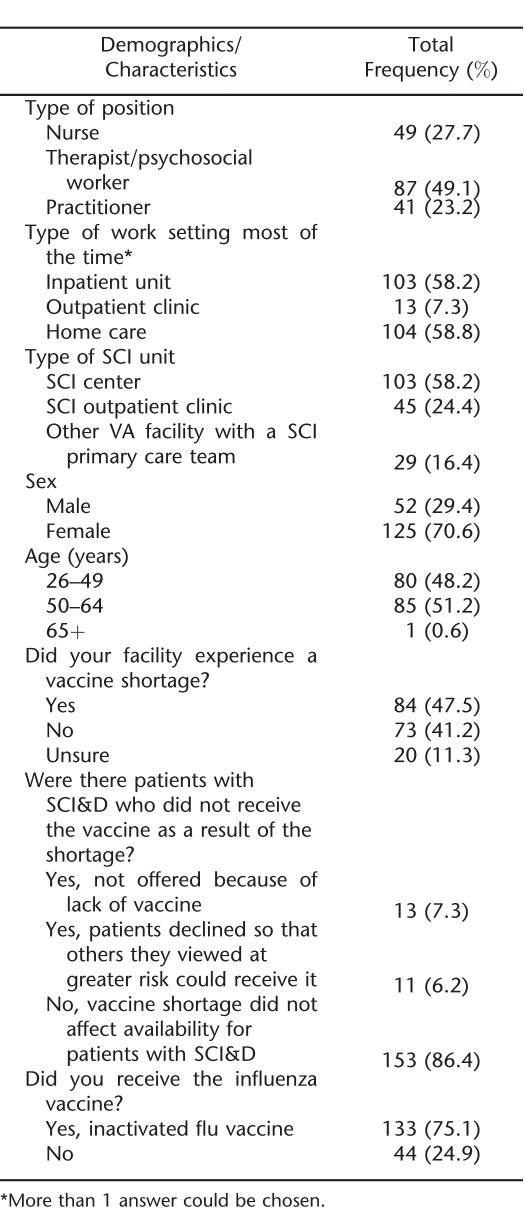
One hundred seventy-seven providers responded to the survey (Table 3). Most worked most of the time in inpatient or home care settings, and more than one half worked in a VA SCI center. The majority of respondents were women, and one half were from 50 to 64 years old. The self-reported vaccination rate in providers was 75.1%. Almost one half of providers reported experiencing a vaccine shortage at their facility. However, most providers (86.4%) reported that the vaccine shortage did not affect the availability of vaccine for patients with SCI&D. A small percentage of providers reported that the vaccine was not offered because of lack of the vaccine at their facility. Only 6.2% reported that patients with SCI&D declined the vaccine so that others could receive it.
Table 3.
Provider Survey: Demographics, Characteristics, and Behaviors (n = 177)
Of practitioners (n = 41), most reported diagnosing influenza in persons with SCI&D using clinical symptoms (97.6%), followed by rapid antigen testing (31.7%). Only 4.9% indicated using serology, and 4.9% used polymerase chain reaction for diagnosing influenza. A small percentage of practitioners indicated that they tested for influenza in persons with SCI&D more often than in past years (4.9%), and 19.5% indicated that, while they did test for influenza, it was not different from past years. Most practitioners reported that they do not test for influenza in patients with SCI&D (75.6%).
Key Informant Interviews
Seventeen of 23 SCI centers responded to requests to be interviewed. Of the respondents, 12 reported influenza vaccine shortages at their VA facility; however, patients with SCI&D had priority for influenza vaccine at 11 of these 12 facilities. At 1 VA Medical Center, priority for influenza vaccine was given to veterans with tetraplegia (high-level injury) in October and November; by December, all veterans with SCI&D had priority.
DISCUSSION
Most respondents were aware of the influenza vaccine shortage in the United States during the 2004–2005 influenza season. The self-reported vaccination rate among survey respondents (71.8%) was comparable with VA medical chart review reported rates of 65% for veterans with SCI&D during the same year and just as good as previous years' rates of 68% by medical record review (10). In addition, most of this high-risk population received their vaccine early in the vaccination season. Those who were aware of the vaccine shortage were more likely to be vaccinated than were those who were unaware.
The results may be caused by the continued and additional efforts initiated by the VA SCI SHG and the SCI QUERI to educate patients and providers regarding risks for persons with SCI&D from influenza and the need to be vaccinated in response to the shortage. It also may be attributed to the fact that all veterans in this sample were users of VA care, and VA users have been found to have higher rates of influenza vaccination than either veterans who do not use VA care or nonveterans (11).
This high vaccination rate is contrary to what has been reported in the general population, where significant decreases in vaccination rates were seen between the 2003–2004 and 2004–2005 influenza seasons (2–5). In particular, persons 50 to 64 years old experienced a significant decrease in vaccination coverage from 52.4% to 28.1% between the 2003–2004 and 2004–2005 seasons (4). In our survey, 73.7% of veterans with SCI&D 50 to 64 years of age were vaccinated. Although we could not directly compare our vaccination rate to the previous year's self-reported rate because of separate sampling strategies, we were able to make some inferences. In our previous research focused on improving influenza vaccination rates in persons with SCI&D, patient self-reported influenza vaccination rates increased between 1999 and 2004, ranging from 57% to 79% (6,8,12,13), and medical chart review reported rates for the same time period ranged from 28% to 68% (10). From the 2001 to 2004 influenza season, vaccination rates increased from 50% to 68% in veterans with SCI&D who were less than 50 years of age, from 61% to 79% for those 50 to 64 years, and from 76% to 87% in those 65 years of age and older (8,13. In this study, the 2004–2005 influenza season vaccination rates for these respective groups were 56%, 74%, and 79%.
Although almost one half of providers reported a vaccine shortage at their facility, this reported shortage had little effect on how providers diagnosed and treated patients with SCI&D during the 2004–2005 influenza season. CDC recommends diagnostic testing to aid in clinical judgment and to guide treatment decisions and also recommends the use of antivirals for early treatment of influenza in persons at high risk (14). The results suggest that an increased awareness and use of these guidelines is needed for this patient population.
The shortage does not seem to have affected the availability of vaccine for persons with SCI&D seen in the vast majority of centers. Data from key informant interviews showed that, although a shortage of influenza vaccine occurred at 12 VA medical centers with an associated SCI center, patients with SCI&D had priority for the vaccine at 11 of 12 SCI centers throughout the vaccination period. Although the number is low, it is unfortunate that some veterans with SCI&D chose to forego the vaccine because they perceived themselves to be at less risk than other individuals. The VA identifies all veterans with SCI&D as high risk and recommends influenza vaccine to the entire population. The CDC recently recommended that any person with a condition that could compromise respiratory function should be vaccinated; this included those with SCI&D (14).
The vaccination rate in providers was also high (75.1%). This is higher than in previous years, in which health care workers treating veterans with SCI&D had a influenza vaccination rate of approximately 50% in the 2001–2002 season (7) and 2002–2003 season (15). This may indicate that providers were more aware of the risk they posed to their patients if unvaccinated, particularly because a national shortage was likely, and some at-risk patients may have gone unvaccinated. However, previous studies had larger sample sizes, the surveys were mailed to providers with follow-up mailings, and the type of provider responding varied. Thus, comparison across years should be made with caution.
Influenza testing by providers did not seem to change, nor were treatment strategies affected by the vaccine shortage. This suggests further emphasis is needed on appropriate use of diagnostic strategies for identifying influenza and treatment options, particularly during likely times of increased circulation of influenza.
There are several limitations to this study. The patient and provider surveys only asked about vaccination during the 2004–2005 season and not about previous seasons; thus, we were unable to directly compare vaccination behavior over time. Second, the response rate for the patient survey was low. The patient survey was a onetime anonymous mailing. Although this was intended as a quality improvement project, repeated mailings may have improved the response rate. Although we could not assess an accurate response rate, low participation in the provider survey may have been caused by the non-specificity of identifying providers using e-mail distribution lists and/or the electronic format of the survey, where some providers may have been less likely to complete the survey than other groups because of limited computer access. In addition, the sampling frame for providers was different from previous surveys (ie, SCI center staff vs SCI center plus SCI clinic staff at non-SCI centers); thus, it is difficult to determine whether there are any biases in our respondent group. Demographic information was not available for nonrespondents for both the patient and provider surveys, so we could not compare how respondents and nonrespondents may have differed.
In this study, there were slightly more non-Hispanic white patient respondents (78%) than in the population reported by LaVela et al (69%) (16). However, the patient respondents were similar in age, sex, marital status, and level of injury to veterans with SCI&D in general. Although this sample of providers was similar to that of a previous survey of providers concerning influenza vaccination in veterans with SCI&D with respect to age and sex and working in an inpatient setting, there were also differences. In the current sample, home care also was a frequent work setting, and therapists, psychologists, and social workers were the predominant responders, with almost equal proportions of nurses and practitioners responding. This is contrary to a survey where only 5% of responders worked most of the time in home care, and most responders were nurses, followed by therapists, psychologists, or social workers (7). The earlier sample was limited to providers at SCI centers, whereas the current sample also included those at non-SCI center facilities, which might account for some of these differences. Non-SCI facilities typically have a physician, nurse, and social worker making up the core of the SCI clinic staff at these sites. Also, LaVela et al (7) used an anonymous mailing compared with the electronic format used in this study, which may explain differences in terms of respondent types because of computer access.
Finally, vaccination coverage was self-reported and subject to misclassification and recall bias. However, previous work has shown exceptional agreement between self-report of vaccination and medical records (17,18).
CONCLUSION
Although most patients were aware of the vaccine shortage, the vaccination rate remained high compared with previous years because VA providers and facilities targeted SCI&D as a high-risk group and prioritized use of the limited vaccine supply. Emphasis should continue educating persons with SCI&D to get vaccinated every influenza season, ensuring the vaccine is available to high-risk populations, and informing providers about the benefits of antiviral agents in treating influenza.
Footnotes
This material is based on work supported by the Office of Research and Development, Health Services Research and Development Service, Quality Enhancement Research Initiative of the Department of Veterans Affairs (SCI 98-000 and SCT 01-169). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. There are no potential conflicts of interest with any author.
REFERENCES
- Centers for Disease Control and Prevention Interim influenza vaccination recommendations, 2004–05 influenza season, October 5, 2004. Morbid Mortal Wkly Rev. 2004;53:1183–1184. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Experiences with obtaining influenza vaccination among persons in priority groups during a vaccine shortage-United States, October-November, 2004. Morbid Mortal Wkly Rev. 2004;53:1153–1155. [PubMed] [Google Scholar]
- DesRoches CM, Blendon RJ, Benson JM. Americans' responses to the 2004 influenza vaccine shortage. Health Aff. 2005;24:822–831. doi: 10.1377/hlthaff.24.3.822. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Influenza vaccination coverage among persons aged 50–64 years enrolled in commercial managed health care plans—United States, 2003–04 and 2004–05 influenza season, September 23, 2005. Morbid Mortal Wkly Rev. 2005;54:921–923. [PubMed] [Google Scholar]
- Link MW, Ahluwalia IB, Euler GL, Bridges CB, Chu SY, Wortley PM. Racial and ethnic disparities in influenza vaccination coverage among adults during the 2004–2005 season. Am J Epidemiol. 2006;163:571–578. doi: 10.1093/aje/kwj086. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Goldstein B, Evans C, et al. Increasing influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2003;26:210–218. doi: 10.1080/10790268.2003.11753684. [DOI] [PubMed] [Google Scholar]
- LaVela SL, Smith B, Weaver FM, Legro MW, Goldstein B, Nichol K. Attitudes and practices regarding influenza vaccination among healthcare workers providing services to individuals with spinal cord injuries and disorders. Infect Control Hosp Epidemiol. 2004;25:933–940. doi: 10.1086/502323. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Smith B, LaVela SL, et al. Interventions to increase influenza vaccination rates for veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10–19. doi: 10.1080/10790268.2007.11753908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CT, LaVela SL, Smith B, Miskevics S, Weaver FM, Goldstein B. Influenza diagnosis and treatment in veterans with spinal cord injury. Arch Phys Med Rehabil. 2006;87:291–293. doi: 10.1016/j.apmr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Veterans Health Administration Office of Quality and Performance, Performance Measurement Reports. Available at: www.oqp.med.va.gov/oqp_services/performance_measurement/dushom.asp. Accessed June 26, 2006.
- Chi R, Reiber GE, Neuzil KM. Influenza and pneumococcal vaccination in older veterans: results from the Behavioral Risk Factor Surveillance System. J Am Geriatr Soc. 2006;54:217–223. doi: 10.1111/j.1532-5415.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- Evans C, Legro M, Weaver FM, Goldstein B. Perceptions about influenza vaccinations among veterans with spinal cord injury. J Spinal Cord Med. 2003;26:204–209. doi: 10.1080/10790268.2003.11753683. [DOI] [PubMed] [Google Scholar]
- LaVela SL, Weaver FM, Goldstein B, Hammond M. Paper presented at the Academy Health Annual Research Meeting; Boston, MA: June 26–28, 2005. Respiratory health in spinal cord injury. Invited Panel: lessons learned from VA Implementation Research. [Google Scholar]
- Centers for Disease Control and Prevention Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices. Morbid Mortal Wkly Rev. 2005;54:1–41. [Google Scholar]
- LaVela SL, Legro M, Weaver FM, Smith B. Staff influenza vaccination: lessons learned? SCI Nurs. 2004;21:153–157. [PubMed] [Google Scholar]
- LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Sci Med. 2004;59:2387–2399. doi: 10.1016/j.socscimed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Korn JE, Baum P. Estimation of outpatient risk characteristics and influenza vaccination status: validation of a self-administered questionnaire. Am J Prev Med. 1991;7:199–203. [PubMed] [Google Scholar]
- Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine. 2003;21:1486–1491. doi: 10.1016/s0264-410x(02)00700-4. [DOI] [PubMed] [Google Scholar]