Abstract
Chemoattractants and chemokines, such as interleukin 8 (IL-8), are defined by their ability to induce directed cell migration of responsive cells. The signal transduction pathway(s) leading to cell migration remain ill defined. We demonstrate that phosphatidylinositol-3-kinase (PI3K) activity, as determined by inhibition using wortmannin and LY294002, is required for IL-8-induced cell migration of human neutrophils. Recently we reported that IL-8 caused the activation of the Ras/Raf/extracellular signal-regulated kinase (ERK) pathway in human neutrophils and that this activation was dependent on PI3K activity. The regulation of cell migration by IL-8 is independent of ERK kinase and ERK activation since the ERK kinase inhibitor PD098059 had no effect on IL-8-induced cell migration of human neutrophils. Additionally, activation of p38-mitogen-activated protein kinase is insufficient and activation of c-Jun N-terminal kinase is unnecessary to induce cell migration of human neutrophils. Therefore, regulation of neutrophil migration appears to be largely independent of the activation of the mitogen-activated protein kinases. The data argue that PI3K activity plays a central role in multiple signal transduction pathways within the human neutrophil leading to distinct cell functions.
Keywords: chemotaxis, wortmannin, c-Jun N-terminal kinase
Cell migration induced by chemoattractants and chemokines is a hallmark of neutrophil activation, as well as a necessary process in a variety of other cell systems including embryogenesis and wound repair. Cell migration can be characterized by three forms of locomotion: (i) random, (ii) kinesis, and (iii) chemotaxis. Random motion occurs in the absence of any environmental stimulus. Kinesis, specifically chemokinesis, is random motion that is influenced by a chemical stimulus. Chemotaxis is directed motion toward a gradient of a stimulus. The mechanical process of migration includes cell polarization, the formation of lamellipodia and filipodia and the attachment of the cell to the surface on which it is migrating (1, 2). Actin filaments and their associated myosin motors drive, while cell surface integrins guide this mechanical process. Data from a variety of laboratories have implicated several signal transduction molecules in the regulation of migration. The small molecular weight GTP binding proteins Rho (3), Rac (4) and Cdc42 (5, 6) regulate the formation of focal adhesions, lamellipodia and filipodia, respectively. Additionally, focal adhesion kinase (1), phosphatidylinositol-3-kinase (PI3K) (7–12) and phospholipase C with its effect on intracellular calcium (7, 13, 14) have been implicated in the activation of cell migration. How these various signaling molecules are integrated to convert the recognition of chemoattractants and chemokines by their receptors into the physical movement of the cell remains undefined.
We and others have previously shown that the receptors for the chemoattractants fMet-Leu-Phe (15–17) and C5a (16, 18) and the chemokine interleukin 8 (IL-8) (16, 19) expressed on neutrophils activate extracellular signal-regulated kinase (ERK) via the Ras regulation of Raf and ERK kinase (MEK). Furthermore, we have shown that the activation of ERK in neutrophils requires PI3K at the level of Ras/Raf (19). With the discovery of additional mitogen-activated protein kinase (MAPK) pathways, including c-Jun N-terminal kinase (JNK) (20, 21) and p38-MAPK (22–24), the question arises whether all of these pathways are activated by a particular stimulus and which if any of these pathways are involved with the activation of specific cellular functions such as migration. This question is particularly important for differentiated cells such as the neutrophil where unregulated tissue infiltration contributes to human disease.
In this report, we address this issue by determining the activation profiles for the known MAPK enzymes in primary human neutrophils stimulated by the chemokine IL-8, as well as, determining the role that these enzymes have in the induction of cell migration. The results indicate that activation of PI3K is required for the induction of neutrophil migration in response to IL-8, but the multiple MAPKs activated in neutrophils in response to IL-8 have no demonstrable role in the induction of neutrophil migration.
MATERIALS AND METHODS
Materials.
Recombinant, human IL-8, the 72-amino acid form, was purchased from R & D Systems. Wortmannin was purchased from Sigma. Recombinant, human tumor necrosis factor α (TNF-α) was purchased from Calbiochem. PD098059 was a generous gift from Alan Saltiel (Parke–Davis Pharmaceutical Research). LY294002 was a generous gift of Kelly Hippen (National Jewish Center). SK&F 86002 (25) was provided by SmithKline Beecham. Blind well Boyden chambers (Nuclepore) were a kind gift of Priscilla Campbell (National Jewish Center). Polyclonal rabbit anti-p38-MAPK sera were raised against a peptide derived from the C-terminal sequence of murine p38-MAPK (22) ATF-2 (1–110 amino acids) was expressed in Escherichia coli and purified as described (26).
Isolation and Preparation of Human Neutrophils.
Neutrophils were isolated from healthy, human immunodeficiency virus-negative blood donors using the method of Haslett et al. (27). Neutrophils were used in Krebs–Ringer phosphate buffer containing 0.2% dextrose and 0.25% human serum albumin (KRPD-HSA) for all assays. For enzyme activity assays, neutrophils were treated with 1 mM phenylmethylsulfonyl fluoride, 0.01 unit/ml aprotinin and 5 μg/ml leupeptin for 30 min at 37°C prior to their use in the various assays.
ERK Assay.
ERK activation in human neutrophils was measured as described (19).
p38-MAPK Assay.
p38 MAPK was assayed by an immune complex kinase assay. Briefly, neutrophils, 4 × 107 per sample, were stimulated with the indicated concentration of cytokine for 10 min at 37°C. The neutrophils were then isolated by centrifugation (200 × g) and resuspended in 500 μl of lysis buffer containing 20 mM Tris·HCl (pH 7.5), 1% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 20 mM NaF, 0.2 mM NaVO3, 1 mM EDTA, 1 mM EGTA, and 5 mM phenylmethylsulfonyl fluoride. Following centrifugation (10,000 × g) to remove nuclei and debris, p38-MAPK was immunoprecipitated from each sample using polyclonal rabbit anti-p38-MAPK sera and protein A Sepharose beads. Following the immunoprecipitation reaction, the beads were washed three times in lysis buffer and twice in kinase buffer containing 25 mM Hepes (pH 7.4), 25 mM β-gylcerophosphate, 25 mM MgCl2, 2 mM dithiothreitol, and 0.1 mM Na3VO4. The kinase reaction was started by adding 40 μl of kinase buffer containing 5 μCi (1 Ci = 37 GBq) of [32P]ATP, and ATF-2 (1–110 amino acids) as substrate. After 30 min at 30°C, the reaction was stopped with 5× Laemmli sample buffer. Samples were then boiled and subjected to SDS/PAGE followed by autoradiography and PhosphorImager (Molecular Dynamics) analysis.
Measurement of Neutrophil Migration.
A standard micropore filter assay using blind well Boyden chambers was performed. Briefly, the lower well of the chamber was filled with KRPD-HSA with or without cytokine. The well was then overlaid with an 8-μm pore size, 150-μm-thick nitrocellulose filter (Sartorius) prewetted in KRPD-HSA. The upper well was screwed down onto the filter and 105 neutrophils in 200 μl were added to the upper well. The chambers were then incubated in a humidified, CO2 incubator at 37°C for 1–2 hr. Following incubation, the filters were harvested and fixed in 70% ethanol. The filters were then stained with hematoxylin, dehydrated, cleared, and mounted as described (28). All samples were tested in triplicate with three independent cell counts taken per replicate filter at ×400 magnification and at a specific depth of field from the top of the filter for a total of nine readings per sample.
Measurement of Intracellular Calcium Concentration.
[Ca2+]i was measured as previously described (29).
Statistical Analysis.
P values were calculated by comparing control versus individual treated samples in each data set using Student’s t test performed using jmp statistical software from SAS Institute (Cary, NC). P values of <95% confidence were rejected as statistically not significant.
RESULTS
IL-8 Activates ERK and p38-MAPK but Not JNK in Neutrophils.
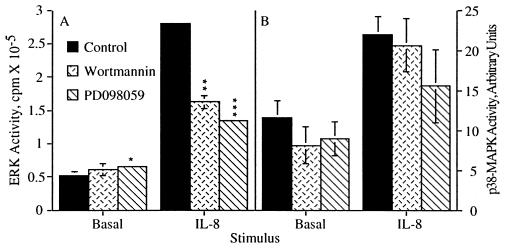
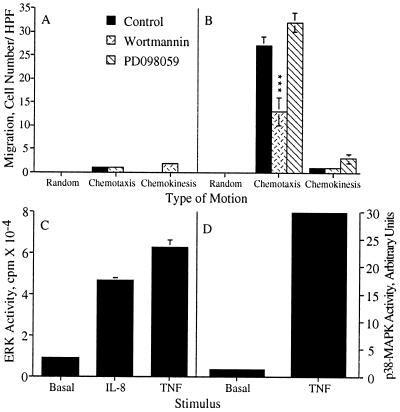
As we recently demonstrated (19), treatment of human neutrophils with the chemokine IL-8 stimulated the activation of ERK (Fig. 1A). Furthermore, this activation was sensitive to the PI3K inhibitor wortmannin (Fig. 1A), as well as the MEK-1 and MEK-2 inhibitor PD098059 (30) (Fig. 1A). These data indicate that the activation of ERK by IL-8 is dependent on the activation of PI3K and MEK. Recently, a second MAPK, p38-MAPK, which is activated by inflammatory cytokines and environmental stress was identified (24). Fig. 1B shows that p38-MAPK in human neutrophils was activated by IL-8. Not surprisingly, the activation of p38-MAPK was distinct from ERK activation; it was not sensitive to either wortmannin or PD098059 (Fig. 1B), at doses of these inhibitors that gave maximal inhibition of ERK, indicating that neither PI3K nor MEK-1 or MEK-2 is involved in p38-MAPK activation by IL-8. Like p38-MAPK, JNK (20), another member of the MAPK family, is activated by inflammatory cytokines and environmental stress (31–33). However, IL-8 did not stimulate JNK activation in human neutrophils (data not shown). Therefore, IL-8 shows selective activation of MAPK pathways in human neutrophils.
Figure 1.
IL-8 stimulated activation of ERK but not p38-MAPK is sensitive to wortmannin and PD098059. Human neutrophils were treated with either the carrier dimethyl sulfoxide alone, 100 nM wortmannin for 10 min or 50 μM PD098059 for 1 hr prior to stimulation with 25 nM IL-8. (A) Samples were stimulated for 3 min then assayed for ERK activity. The data shown represent the mean ± SEM of triplicate measurements of ERK activity for each sample and are representative of more than six independent experiments. ∗, P = 0.0126; ∗∗, P = 0.0066; ∗∗∗, P = 0.0003. (B) Samples were stimulated for 10 min then assayed for p38-MAPK activity. The data shown represent the mean ± SEM of PhosphorImager data from five independent experiments.
PI3K Activity Regulates IL-8-Stimulated Chemotaxis Independent of MEK and ERK.
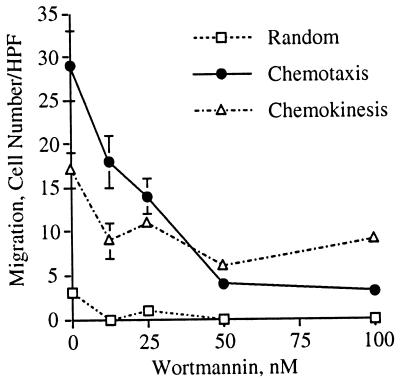
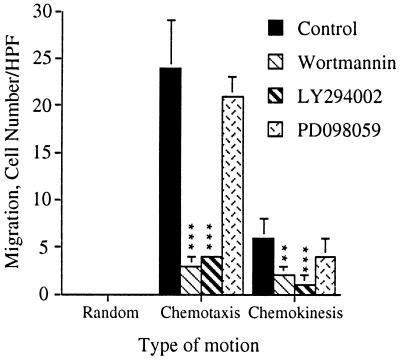
ERK has been implicated in gene transcription, cell growth and differentiation (34) and as a negative regulator of apoptosis (35). We therefore investigated its role in the induction of neutrophil migration in response to IL-8. Since ERK activation was sensitive to both wortmannin and PD098059 that act on different proteins in the signal transduction pathway leading to ERK activation, we tested their effect on IL-8-induced neutrophil migration. Wortmannin inhibited neutrophil chemotaxis in a dose dependent manner with an IC50 of 26 nM (Figs. 2 and 3). Wortmannin also inhibited IL-8-induced neutrophil chemokinesis (Figs. 3 and 4). PD098059, on the other hand, did not inhibit neutrophil chemotaxis or chemokinesis (Fig. 4). Because the specificity of wortmannin, at low doses, was recently questioned (36), we also tested the ability of LY294002 (37), a competitive inhibitor of PI3K, to inhibit cell migration. LY294002, like wortmannin, inhibited neutrophil chemotaxis and chemokinesis (Fig. 4). Therefore, the inhibition of neutrophil chemotaxis by wortmannin is due to an inhibition of PI3K. These data indicate that PI3K activity is required for the induction of both chemotaxis and chemokinesis in primary human neutrophils stimulated by IL-8. Furthermore, IL-8-induced activation of MEK and subsequent activation of ERK, which are regulated by PI3K, are not required for neutrophil migration. Together, these data indicate that PI3K regulates chemotaxis independent of its regulation of ERK activation.
Figure 2.
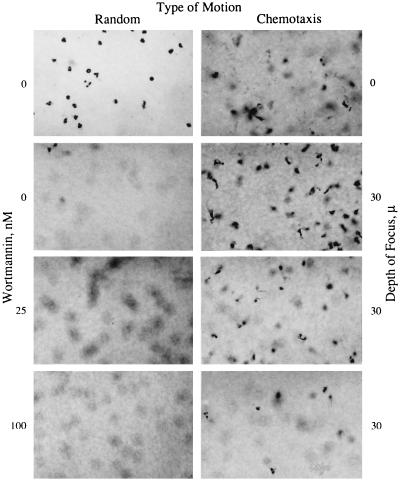
Wortmannin inhibits IL-8-induced cell migration of human neutrophils. Freshly isolated neutrophils were treated with the indicated concentrations of wortmannin or carrier alone for 10 min prior to their use in the chemotaxis assay. Random motion was measured in the presence of KRPD-HSA only. Chemotactic motion was measured in the presence of KRPD-HSA containing 2.5 nM IL-8 (a maximal dose for neutrophil chemotaxis, data not shown) in the lower chamber of the blind well Boyden chamber. Migration was measured at a depth of 30 μm. The photomicrographs shown are of individual filters representative of triplicate filters analyzed for each sample. (×400.)
Figure 3.
Wortmannin inhibits IL-8-induced cell migration with an IC50 of 26 nM. This figure shows the numerical analysis of the chemotaxis assay presented in Fig. 2. Chemokinetic motion was measured in the presence of KRPD-HSA containing 2.5 nM IL-8 in both the lower and upper chambers of the blind well Boyden chamber. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (three fields from each replicate filter) at a depth of 30 μm from the top of the filter.
Figure 4.
Both wortmannin and LY294002 but not PD098059 inhibit IL-8-induced cell migration. Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min, 50 μM LY294002 for 5 min or 50 μM PD098059 for 1 hr prior to their use in the chemotaxis assay. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (3 fields from each replicate filter) at a depth of 60 μm from the top of the filter and are representative of at least three independent experiments. ∗∗∗, P < 0.0001; ∗∗, P = 0.0064. The assays were performed as described in the legends to Figs. 2 and 3 and in Materials and Methods.
Wortmannin and PD098059 Do Not Inhibit IL-8-Induced Calcium Mobilization Nor Effect Cell Viability.
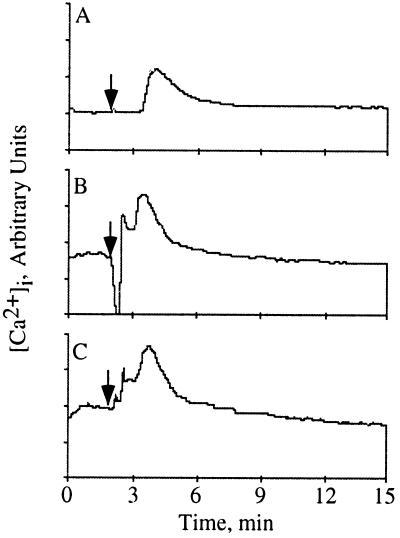
To verify that wortmannin and PD098059 were not having a global effect on signal transduction in the neutrophil, we tested their effect on calcium mobilization stimulated by IL-8. As previously demonstrated (38), IL-8 stimulated the mobilization of intracellular calcium in human neutrophils (Fig. 5A). This mobilization was not inhibited by either wortmannin (Fig. 5B) or PD098059 (Fig. 5C). The data suggest that PI3K activity does not influence IL-8-induced calcium mobilization. Additionally, none of the inhibitors used in these studies had any effect on cell viability as determined by trypan blue exclusion (data not shown). Therefore, wortmannin and PD098059 were not inhibiting IL-8 stimulated signal transduction indiscriminately as measured by calcium mobilization and p38-MAPK activation.
Figure 5.
Wortmannin and PD098059 do not inhibit IL-8 stimulated calcium mobilization. Human neutrophils were loaded with 5 μM Indo-1 AM for 1 hr and simultaneously incubated with either carrier alone (A), 100 nM wortmannin for the final 10 min (B) or 50 μM PD098059 for 1 hr (C). [Ca2+]i was determined by flow microfluorimetry using a cytofluorograf 50H (Ortho Diagnostics) with acqcyte software (Phoenix Flow Systems, San Diego). Samples were analyzed for 2 min prior to stimulation to establish the basal [Ca2+]i. IL-8, 25 nM in KRPD-HSA was then added to the sample, indicated by the arrow, and data collection was continued for an additional 13 min. The data was analyzed using mtime software (Phoenix Flow Systems). The data shown are representative of five independent experiments. The increased resting levels of calcium in B and C are due to incomplete removal from the flow cytometer of IL-8 used to stimulate the previous sample as determined by analyzing the samples in different orders (data not shown).
Activation of p38-MAPK Is Insufficient for the Induction of Chemotaxis.
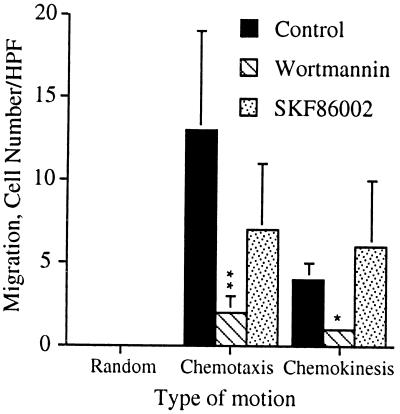
Because neither wortmannin nor PD098059 had any effect on IL-8-induced p38-MAPK activation, we needed an alternate method to address the role, if any, of p38-MAPK in cell migration. We tested the ability of TNF-α, a strong activator of p38-MAPK in epithelial cells (24), to induce cell migration in human neutrophils. TNF-α did not stimulate cell migration of human neutrophils (Fig. 6A) even though the cells responded well to IL-8 stimulation (Fig. 6B). However, TNF-α did strongly activate both ERK (Fig. 6C) and p38-MAPK (Fig. 6D) in human neutrophils. Therefore, the data indicate that activation of p38-MAPK and ERK are insufficient to induce cell migration. We also tested the effect of the p38-MAPK inhibitor, SK&F 86002 (25), on IL-8-induced cell migration in human neutrophils. SK&F 86002 had no statistically significant effect on either IL-8-induced chemotaxis or chemokinesis even though wortmannin completely inhibited these responses in the human neutrophils (Fig. 7).
Figure 6.
TNF does not stimulate cell migration of human neutrophils but does stimulate ERK and p38-MAPK activation. (A and B) Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min or 50 μM PD098059 for 1 hr prior to their use in the chemotaxis assay. Random motion was measured in the presence of KRPD-HSA only. Chemotactic motion was measured in the presence of KRPD-HSA containing either 10 ng/ml TNF (A) or 2.5 nM IL-8 (B) in the lower chamber of the blind well Boyden chamber. Chemokinetic motion was measured in the presence of KRPD-HSA containing either 10 ng/ml TNF (A) or 2.5 nM IL-8 (B) in both the lower and upper chambers of the blind well Boyden chamber. The data shown represent the mean ± SEM of cell counts taken from nine high power fields (three fields from each replicate filter) at a depth of 60 μm from the top of the filter. ∗∗∗, P = 0.0005. (C and D) Samples were stimulated with 10 ng/ml TNF for 10 min and then assayed for ERK activity (C) or p38-MAPK activity (D). (C) The data shown represent the mean ± SEM of triplicate measurements of ERK activity for each sample. (D) The PhosphorImager data shown are representative of at least three independent experiments.
Figure 7.
SK&F 86002 does not inhibit IL-8-induced cell migration. Human neutrophils were treated with either carrier alone, 100 nM wortmannin for 10 min, or 10 μM SK&F 86002 for 1 hr prior to their use in the chemotaxis assay. The data shown represent the mean ± SEM of the mean cell counts from three independent experiments performed and analyzed as described in the legend to Figs. 2, 3, 4 and Materials and Methods. ∗∗, P = 0.0041; ∗, P = 0.0198.
DISCUSSION
We recently demonstrated that the activation of ERK in human neutrophils by IL-8 occurs through the Ras/Raf/MEK pathway and is dependent on PI3K activation (19). We have now shown that the MAPK family member p38-MAPK, but not JNK, is also activated by IL-8 stimulation of neutrophils. This represents the first demonstration of the activation of p38-MAPK by a heterotrimeric G protein-coupled chemoattractant/chemokine receptor system. Unlike ERK activation, the activation of p38-MAPK by IL-8 does not require the activation of PI3K or MEK since neither wortmannin nor PD098059 had any effect on p38-MAPK activation. The exact signal transduction pathway leading from IL-8 stimulation to p38-MAPK activation in the human neutrophil remains undefined. The small GTP binding proteins Rac and Cdc42 through their activation of p21-activated kinase have been implicated as upstream regulators of p38-MAPK in transformed cells (39, 40). Furthermore, MAPK kinase-3 (41), MAPK kinase-4 (42), and MAPK kinase-6 (41) have been shown to phosphorylate and activate p38-MAPK. Whether IL-8 activates p38-MAPK in human neutrophils through Rac/Cdc42, p21-activated protein kinase and MAPK kinase remains to be determined.
The lack of JNK activation in response to IL-8 stimulation defines the selective regulation of MAPK pathways by IL-8. Whether this selective regulation results from expression of specific signal transduction molecules or some other mechanism in human neutrophils remains to be determined. MEK kinases have previously been shown to lie upstream of JNK activation (43, 44). Preliminary data in our laboratory suggests that although multiple MEK kinases are expressed in human neutrophils they are not measurably activated by IL-8. Therefore, the lack of measurable JNK activation may reflect, in part, the failure of IL-8 to activate upstream kinases that lie in the JNK pathway in human neutrophils.
Cell migration plays a key role in a variety of cell responses including embryogenesis, inflammation, wound repair and metastasis. This process requires the coordinated regulation and integration of multiple steps including cell polarization, membrane extension, adhesion, contraction, and release (1, 2). The biochemical reactions resulting in these steps are just now beginning to be elucidated. The results described herein represent a first attempt to correlate the activation of specific kinase pathways with the induction of chemotaxis in a primary human cell population, specifically neutrophils. We have now shown that IL-8 stimulated MEK activation with its subsequent activation of ERK is not required for IL-8-induced chemotaxis or chemokinesis of human neutrophils. Likewise, the lack of chemotaxis in the presence of p38-MAPK activation in neutrophils stimulated with TNF-α suggests that activation of p38-MAPK is insufficient for the induction of cell migration. Additionally, the lack of a significant effect on both chemotaxis and chemokinesis by the p38-MAPK inhibitor SK&F 86002 indicates that neutrophil cell migration induced by IL-8 is not critically regulated by p38-MAPK. Our data do indicate that PI3K activity is required for the induction of chemotaxis and chemokinesis in human neutrophils stimulated by IL-8. We previously showed that functional PI3K was also required for Ras regulation of Raf in the pathway leading to ERK activation by IL-8 (19). Together, these data suggest that PI3K activity contributes to the regulation of several complex regulatory pathways in the human neutrophil.
The fact that PI3K activity is required for the induction of neutrophil chemotaxis in response to a gradient of IL-8 but MEK and ERK are not suggests a bifurcation of this signal transduction pathway at the level of PI3K. This bifurcation could simply reflect the requirement for products of the PI3K reaction for the generation of independent signals leading to cell migration and ERK activation. Alternatively, the bifurcation could be occurring at the level of the class of PI3K, p85/p110 PI3K vs. PI3Kγ, associated with each pathway, as well as the isoform of the p85/p110 PI3K activated (45). Both p85/p110 PI3K and PI3Kγ are known to be activated in neutrophils by IL-8 (46, 47). Furthermore, p85/p110 PI3K and PI3Kγ are differentially regulated by protein tyrosine kinases (48) and heterotrimeric G proteins (46, 47), respectively, and differ in their interactions with other proteins. Finally, the production of multiple forms of D-3 phosphoinositides by PI3K (45), and the identification of multiple potential targets for PI3K activity including the nonclassical forms of PKC (49, 50) and the Rho family of small GTP binding proteins (7, 8) add additional levels of complexity to PI3K regulated pathways. How these various levels of complexity impact on our observation that PI3K activity is required for chemotaxis in human neutrophils remains to be determined.
The role of PI3K activity in the regulation of actin assembly that is required for cell migration remains controversial. Previous studies have suggested that PI3K activity is not required for the regulation of actin assembly (9, 51–53). However, these studies only investigated total F-actin content, not the specific forms of actin assembly that we now know are differentially regulated by Rho (3), Rac (4), and Cdc42 (5, 6). In contrast, work with the platelet-derived growth factor receptor β suggests that PI3K activation is required for the induction of actin rearrangements (7, 8). Furthermore, the relationship of PI3K to Rho, Rac, and Cdc42 also remains controversial. Recent data have suggested that PI3K activity is upstream (7, 8), downstream (54–56), or independent (56) of Rho, Rac, and Cdc42. These seemingly contradictory observations most likely reflect a nonlinear relationship between PI3K activity and the small molecular weight GTP binding proteins. Additionally, these data may also reflect the selective activation of different classes of PI3Ks. Therefore, the role of PI3K activity in chemotaxis may be multifaceted especially in a cell type such as neutrophils that express and activate both p85/p110 PI3K and PI3Kγ. This complexity is also reflected in a previous report that suggested that PI3K activity is required for neutrophil migration in response to growth factors but not to chemoattractants and chemokines (57). Nevertheless, the data argue that PI3K activity has a critical role in integrating the signals controlling chemotaxis and other functions in the human neutrophil. Clarification of this role awaits further investigation of the individual functions of p85/p110 PI3K and PI3Kγ, and perhaps more importantly their regulators and targets.
Acknowledgments
This work was supported by National Institutes of Health Grants GM30324, DK37871, DK48845 (G.L.J.), HL34303 (G.S.W.), and HL09640 (C.K.) and by a research grant from the University of Colorado Cancer Center (C.K.).
ABBREVIATIONS
- PI3K
phosphatidylinositol-3-kinase
- IL-8
interleukin 8
- ERK
extracellular signal-regulated kinase
- MEK
ERK kinase
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- TNF-α
tumor necrosis factor α
- KRPD-HSA
Krebs–Ringer phosphate buffer containing dextrose and human serum albumin
References
- 1.Lauffenburger D A, Horwitz A F. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison T J, Cramer L P. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 3.Ridley A J, Hall A. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 4.Ridley A J, Paterson H F, Johnston C L, Diekman D, Hall A. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 5.Kozma R, Ahmed S, Best A, Lim L. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobes C D, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 7.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Nature (London) 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 8.Wennstrom S, Siegbahn A, Yokote K, Arvidsson A-K, Heldin C-H, Mori S, Claesson-Welsh L. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- 9.Kovacsovics T J, Bachelot C, Toker A, Vlahos C J, Duckworth B, Cantley L C, Hartwig J H. J Biol Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]
- 10.Hartwig J, Bokoch G M, Carpenter C L, Janmey P A, Taylor L A, Toker A, Stossel T P. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 11.Derman M P, Cunha M J, Barros E J G, Nigam S K, Cantley L G. Am J Physiol. 1995;268:F1211–F1217. doi: 10.1152/ajprenal.1995.268.6.F1211. [DOI] [PubMed] [Google Scholar]
- 12.Turner L, Ward S G, Westwick J. J Immunol. 1995;155:2437–2444. [PubMed] [Google Scholar]
- 13.Chen P, Xie H, Sekar M C, Gupta K, Wells A. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornfeldt K E, Raines E W, Nakano T, Graves L M, Krebs E G, Ross R. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worthen G S, Avdi N, Buhl A M, Suzuki N, Johnson G L. J Clin Invest. 1994;94:815–823. doi: 10.1172/JCI117401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson H L, Marshall C J, Saklatvala J. J Biol Chem. 1994;269:9486–9492. [PubMed] [Google Scholar]
- 17.Grinstein S, Butler J R, Furuya W, L’Allemain G, Downey G P. J Biol Chem. 1994;269:19313–19320. [PubMed] [Google Scholar]
- 18.Buhl A M, Avdi N, Worthen G S, Johnson G L. Proc Natl Acad Sci USA. 1994;91:9190–9194. doi: 10.1073/pnas.91.19.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knall C, Young S, Nick J A, Buhl A M, Worthen G S, Johnson G L. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 20.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 21.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, Lee J-D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 23.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Liamazares A, Zamanillo D, Hunt T, Nebreda A R. Cell. 1994;78:1–20. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 24.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 25.Lee J C, Young P R. J Leukocyte Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Malek A-H, Heasley L E, Kyriakis J M, Avurch J, Kroll D J, Johnson G L, Hoeffler J P. Mol Endocrinol. 1992;6:2079–2089. doi: 10.1210/mend.6.12.1337144. [DOI] [PubMed] [Google Scholar]
- 27.Haslett C, Guthrie L A, Kopaniak M, Johston R B J, Henson P M. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 28.Hiester A A, Metcalf D R, Campbell P A. Cell Immunol. 1992;139:72–80. doi: 10.1016/0008-8749(92)90100-4. [DOI] [PubMed] [Google Scholar]
- 29.Cambier J, Chen Z-Z, Pasternak J, Ransom J, Sandoval V, Pickles H. Proc Natl Acad Sci USA. 1988;85:6493–6497. doi: 10.1073/pnas.85.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazar D F, Wiese R J, Brady M J, Mastick C C, Waters S B, Yamauchi K, Pessin J E, Cuatrecasas P, Saltiel A R. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 31.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 32.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 33.Kharbanda A, Ren R, Pandey R, Shafman T D, Feller S M, Welchselbaum R R, Kufe D W. Nature (London) 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 34.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 35.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 36.Cross M J, Stewart A, Hodgkin M N, Kerr D J, Wakelam M J O. J Biol Chem. 1995;270:25352–25355. doi: 10.1074/jbc.270.43.25352. [DOI] [PubMed] [Google Scholar]
- 37.Vlahos C J, Matter W F, Hui K Y, Brown R F. J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 38.Thelen M, Peveri P, Kernen P, Von Tscharner V, Walz A, Baggiolini M. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- 39.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 40.Bagrodia S, Derijard B, Davis R J, Cerione R A. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 41.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 43.Lange-Carter C, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 44.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 45.Ward S G, June C H, Olive D. Immunol Today. 1996;17:187–197. doi: 10.1016/0167-5699(96)80618-9. [DOI] [PubMed] [Google Scholar]
- 46.Stephens L, Smrcka A, Cooke F T, Jackson T R, Sterweis P C, Hawkins P T. Cell. 1994;77:83093. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 47.Stoyanov B, Volinia S, Hanck T, Rubio T, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan J J, Waterfield M D, Wetzker R. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 48.Fantl W J, Johnson D E, Williams L T. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi H, Brewer K A, Exton J H. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 50.Toker A, Meyer M, Reddy K K, Falck J R, Aneja R, Aneja S, Parra A, Burns D J, Ballas L M, Cantley L C. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 51.Wymann M P, Kernen P, Bengtsson T, Andersson T, Baggiolini M, Deranleau D A. J Biol Chem. 1990;265:619–622. [PubMed] [Google Scholar]
- 52.Arcaro A, Wymann M P. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlahos C J, Matter W F, Brown R F, Traynor-Kaplan A E, Heyworth P G, Prossnitz E R, Ye R D, Marder P, Schelm J A, Rothfuss K J, Serlin B S, Simpson P J. J Immunol. 1995;154:2413–2422. [PubMed] [Google Scholar]
- 54.Zhang J, King W G, Dillon S, Hall A, Feig L, Rittenhouse S E. J Biol Chem. 1993;268:22251–22254. [PubMed] [Google Scholar]
- 55.Zheng Y, Bagrodia S, Cerione R A. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- 56.Zhang J, Zhang J, Benovic J L, Sagai M, Wetzker R, Gout I, Rittenhouse S E. J Biol Chem. 1995;270:6589–6594. doi: 10.1074/jbc.270.12.6589. [DOI] [PubMed] [Google Scholar]
- 57.Thelen M, Uguccioni M, Bosiger J. Biochem Biophys Res Commun. 1995;217:1255–1262. doi: 10.1006/bbrc.1995.2903. [DOI] [PubMed] [Google Scholar]