Abstract
Background/Objective:
Orthostatic hypotension is a well-defined clinical consequence of spinal cord injury (SCI), particularly in those with tetraplegia. The etiology of orthostatic hypotension is thought to be loss of sympathetic vasomotor control, although other factors may play a role. There is evidence of up-regulation of nitric oxide synthase (NOS) activity after hind-limb suspension in rats, a condition of antigravity that may have similar vascular effects as shown in persons with tetraplegia caused by paralysis. The study objective was to determine the effect of a NOS inhibitor (nitro-L-arginine methyl ester [L-NAME]) on supine mean arterial pressure in persons with chronic tetraplegia compared with non-SCI controls.
Methods:
Fourteen individuals participated (7 with tetraplegia and 7 controls). Subjects visited the laboratory twice for placebo on day 1 and L-NAME (1 mg/kg) on day 2; both were infused intravenously over 60 minutes. Blood pressure was monitored for 3 hours after infusion at the brachial artery using a standard manual cuff.
Results:
Mean arterial pressure (MAP) was lower at baseline (P < 0.05) and after placebo administration (P < 0.0001) in the tetraplegia group compared with the control group. L-NAME increased MAP in both groups; however, the relative increase was greater in the tetraplegia group compared with the control group, such that group differences for MAP were eliminated. Supine MAP was normalized with L-NAME, and there was an increased sensitivity to NOS inhibition in the group with tetraplegia.
Conclusions:
These findings indicate that blood pressure dysregulation in persons with tetraplegia may reflect increased vascular NO and suggest a novel treatment of hypotension using NOS inhibition in this population.
Keywords: Spinal cord injuries, Arterial pressure, Orthostatic hypotension, Nitro-l-arginine methyl ester, Nitric oxide inhibitor, Tetraplegia
INTRODUCTION
Persons with spinal cord injury (SCI) exhibit several cardiovascular abnormalities caused by autonomic dysfunction, including orthostatic hypotension (OH), which is most severe in those with tetraplegia (1). OH is defined by The Consensus Committee of the American Autonomic Society and the American Academy of Neurology as a decrease in systolic blood pressure (SBP) of ≥20 mmHg and/or in diastolic blood pressure (DBP) of ≥10 mmHg with the assumption of an upright posture (2). OH is an appreciated clinical problem during the acute and subacute phases of SCI (3), and in persons with chronic tetraplegia, significant reductions in seated vs supine SBP and DBP were observed that met the criteria of OH (Tuckman et al, 2002, unpublished data).
OH in individuals with chronic SCI is thought to primarily reflect diminution of peripheral sympathetic vasomotor control (1,4,5); however, emerging evidence suggests that upregulation of the potent vasodilator nitric oxide (NO) is also implicated (6,7. Under normal physiological conditions, NO is produced in endothelial cells to modulate vascular tone (8,9 and is induced in spinal nerves after traumatic SCI (10–12). In pathological states, NO may be produced in high concentrations by inflammatory cells, vascular smooth muscle, and endothelial cells (13). Increased release of NO has been associated with orthostatic intolerance after microgravity-induced cardiovascular deconditioning after hind-limb suspension, space travel, and prolonged bed rest (14–17). It has been proposed that upregulation of NOS expression shown in these conditions of microgravity may have similar vascular effects in persons with tetraplegia (7) and may contribute to the increased prevalence of OH.
NO is synthesized by the enzyme NO synthase (NOS), which has 3 isoforms: eNOS (endothelial NOS), nNOS (neuronal NOS), and iNOS (inducible NOS). The use of a nonspecific NOS inhibitor—nitro-L-arginine methyl ester (L-NAME)—has been shown to reduce production of NO through inhibition of all NOS isoforms and increase supine mean arterial pressure (MAP) in normotensive healthy humans (18), but the effects of L-NAME on MAP in persons with underlying disorders of blood pressure regulation is unknown. The objective of this study was to determine the supine MAP response to L-NAME infusion and to determine the relative effect of L-NAME on MAP in individuals with tetraplegia compared with non-SCI controls. We hypothesized that L-NAME would increase MAP and that persons with tetraplegia would show increased sensitivity to NOS inhibition compared with non-SCI controls.
METHODS
Subjects
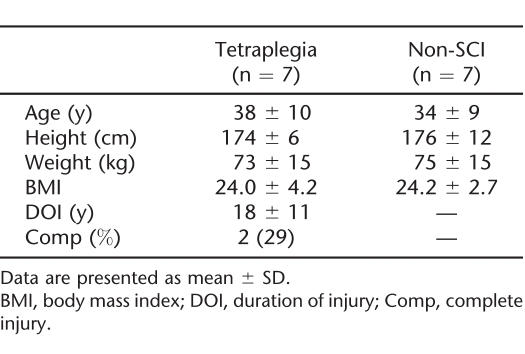
Subjects (n = 14) were men from 22 to 51 years of age, with no known history of cardiovascular disease, pulmonary disease, or diabetes mellitus. All were current nonsmokers for a minimum of 1 year before the study and were not taking medications known to affect autonomic cardiovascular function. Subjects with tetraplegia (n =7) were healthy outpatients, with a minimum of 6 years since injury; all were nonambulatory and capable of maintaining an independent lifestyle. Using the American Spinal Injury Association (ASIA) classification of neurological impairment, 2 individuals were diagnosed with complete injury (ASIA A) and the other 5 were diagnosed with incomplete injury (ASIA B and C). The non-SCI control subjects (n = 7) were matched for age, height, and weight to the subjects with SCI. Approval by the Institutional Review Board for Human Studies was granted, and informed consent was obtained before study initiation.
Protocol Procedures
Subjects were instructed to be well hydrated and to avoid heavy exertion, caffeine, and alcohol for a minimum of 24 hours before testing, and they reported to the laboratory between 8:00 and 9:00 AM on 2 separate days. On arrival, subjects were placed in the supine position and rested quietly while ECG electrodes were applied to the chest for heart rate (HR) monitoring (742 Mennen Medical ECG Monitor, Bio-Medical Equipment Service Co., Louisville, KY). A standard adult blood pressure (BP) cuff was placed around the left upper arm for manual blood pressure recordings, which were obtained by a trained clinician every 15 minutes while the subject remained in the supine position (W.A. Baum Co., Copiague, NY). An intravenous catheter was placed in the right antecubital vein and secured for administration of placebo (normal saline) on day 1 and L-NAME (1 mg/kg; Clinalfa, Läufelfingen, Switzerland) on day 2. After 20 minutes of supine rest, baseline (BL) HR and BP data were collected, and the 60-minute infusion was initiated. Subjects remained in the supine position for 240 minutes: 1 hour of infusion (60 minutes) and 3 hours after infusion (120, 180, and 240 minutes); HR and MAP ([systolic + diastolic + diastolic]/3) data are reported as an hourly mean.
Data Analysis
All continuous variables are reported as mean ± SD. The HR and MAP data were analyzed with separate univariate mixed factorial analyses of variance (ANOVAs; group [tetraplegia, control] by drug [placebo, L-NAME] and by time [BL, 60, 120, 180, 240 minutes]). The Huynh-Feldt correction for violations of the sphericity assumption was used. Significance was set at the 0.05 α level. The authors had full access to the data and take responsibility for their integrity. All authors have read and agree to the manuscript as written.
RESULTS
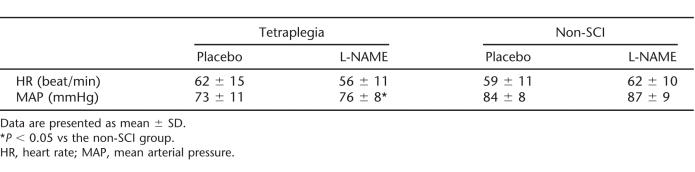
There were no group differences for any demographic parameter (Table 1). Baseline HR and MAP were similar on days 1 and 2, and HR was comparable among the groups; however, baseline MAP was reduced in the tetraplegia group compared with the control group (Table 2). L-NAME infusion was well tolerated by all subjects, and there were no reports of serious adverse events.
Table 1.
Subject Characteristics
Table 2.
Baseline Heart Rate and Blood Pressure
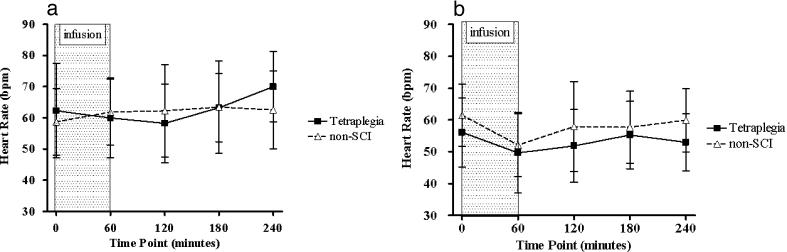
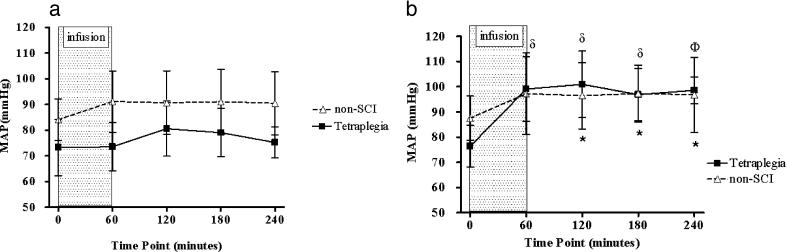
HR was not significantly altered after placebo (Figure 1a) or L-NAME (Figure 1b) administration in either group. The MAP data showed a significant 3-way interaction effect (P = 0.01), which was subsequently decomposed into separate 2-factor group by time analyses (placebo and L-NAME; see Figure 2a and b). For the placebo data (Figure 2a), the interaction effect was not significant. For the L-NAME data, the interaction effect was significant (P = 0.02), and subsequent pairwise comparisons showed that MAP was significantly increased from BL at all time-points after infusion in the group with tetraplegia and at 120, 180, and 240 minutes after infusion in the control group. Change in hourly MAP with L-NAME was greater in the tetraplegia group compared with the non-SCI group at 60 (23 ± 10 vs 10 ± 11 mmHg, respectively; P < 0.05), 120 (25 ± 12 vs 9 ± 9 mmHg, respectively; P < 0.05), 180 (21 ± 12 vs 10 ± 8 mmHg, respectively; P = 0.07), and 240 minutes (22 ± 9 vs 9 ± 9 mmHg, respectively; P < 0.05; Figure 3).
Figure 1. Effect of placebo (a) and L-NAME (b) on heart rate in the tetraplegia (squares) and non-SCI (triangles) groups. There was no significant change in HR after placebo or L-NAME administration over the duration of observation in either group.
Figure 2. Effect of placebo (a) and L-NAME (b) on MAP in the tetraplegia (squares) and non-SCI (triangles) groups. MAP was significantly reduced in the tetraplegia group compared with the non-SCI group during the placebo trial (a: P < 0.0001). After L-NAME administration, there was no group difference for MAP over the duration of observation (b). Non-SCI group: *P < 0.05 vs baseline MAP. Tetraplegia group: δP < 0.01 and ΦP < 0.001 vs baseline MAP.
Figure 3. Percent change in MAP after L-NAME (1 mg/kg) infusion. The increase in hourly MAP (%) was significantly greater in the tetraplegia group compared with the non-SCI group each hour after infusion. *P < 0.05 vs the non-SCI group.

DISCUSSION
In persons with tetraplegia, supine BP was normalized after NOS inhibition, and the BP increase after L-NAME infusion was more pronounced compared with the non-SCI controls. These findings show increased sensitivity to NOS inhibition in persons with chronic tetraplegia, which is consistent with increased vasculature NO production.
After exposure to microgravity, postural intolerance is commonly reported (6,7,14–17,19), and persons with SCI have been likened to models of microgravity because of neuronal compromise and sustained bed rest after injury (7). Several mechanisms have been proposed to explain microgravity-induced orthostatic intolerance. Hypovolemia has been implicated, although volume repletion and fludrocortisone failed to reverse hypotension after spaceflight or hind-limb suspension (20,21. There is also evidence supporting impaired vasoconstrictive capacity in humans and animals after exposure to microgravity, shown by significantly reduced pressor responses to vasoactive substances (16,19,22) and an attenuated plasma norepinephrine response to upright posture associated with lower standing vascular resistances (23,24).
This is the first report to document increased MAP after the administration of a NOS inhibitor in humans with compromised sympathetic cardiovascular control. There are reports of robust increases in supine BP after L-NAME infusion in healthy humans, with peak effects documented 60 to 120 minutes after infusion (18,25. Sustained elevations (after 1 hour) in MAP with L-NAME have been related to increased sympathetic nervous system activity, because the latent effects were attenuated in normotensive subjects with phentolamine (18) and in rats after pharmacologic sympathectomy with guanethidine (26). There are also data indicating that L-NAME administered intracranially reduces NO release in the cardiovascular regulatory center, thereby suppressing tonic inhibition of sympathetic outflow (27). L-NAME is a nonspecific NOS inhibitor, and the precise effects on the vasculature can not be determined; however, the sustained elevation in MAP 240 minutes after infusion in subjects with tetraplegia suggests a mechanism that is independent of the sympathetic nervous system and implicates suppression of vascular NO.
Impaired vasoconstrictor capacity may play a critical role in the development of microgravity-induced OH in persons with SCI, and we recently documented increased iNOS expression in the thoracic aorta of SCI compared with sham-operated rats 14 days after transection (28). Support of this finding comes from evidence of increased vascular NO after hind-limb suspension in rats, an earth-bound model of microgravity (6). In isolated arterial rings of rats exposed to prolonged hind-limb suspension, femoral artery contractile response to norepinephrine was normalized with aminoguanidine, a selective iNOS inhibitor (19). Additional in vivo evidence of increased MAP with aminoguanidine was also noted (19), suggesting that blood pressure dysregulation after exposure to microgravity relates to NO-dependent mechanisms. Indeed, plasma levels of NO were significantly elevated above BL 6 hours after hind-limb suspension, and MAP was maintained on release of suspension in rats treated with 20 mg/kg of L-NAME (15).
There are several limitations that should be considered when interpreting the data reported herein. Neural compromise caused by an SCI is a patho-physiologic state, and application of these findings to other conditions of OH may therefore be imprudent. The study subjects with tetraplegia comprised a small number of individuals with relatively homogeneous injuries, chronic (>1 year after injury), incomplete (71%) cervical lesions between C4 and C6, thus limiting extrapolation of these findings to a more heterogeneous SCI population.
CONCLUSION
From these data, we report heightened sensitivity to NOS inhibition in the group with tetraplegia and suggest that OH is a consequence of increased vascular NO in this model of microgravity. In addition, supine BP was normalized with L-NAME in persons with tetraplegia compared with a healthy, normotensive, age-matched non-SCI control group, which may lead to novel clinical treatment options for OH in this and other conditions of diminished neural vascular control.
Footnotes
This study was supported by the Veterans Affairs Rehabilitation Research and Development Service (#B3203R) and by the United Spinal Association.
REFERENCES
- Blackmer J. Orthostatic hypotension in spinal cord injured patients. J Spinal Cord Med. 1997;20:212–217. doi: 10.1080/10790268.1997.11719471. [DOI] [PubMed] [Google Scholar]
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- Illman A, Stiller K, Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord. 2000;38:741–747. doi: 10.1038/sj.sc.3101089. [DOI] [PubMed] [Google Scholar]
- Barber DB, Rogers SJ, Fredrickson MD, Able AC. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal Cord. 2000;38:109–111. doi: 10.1038/sj.sc.3100959. [DOI] [PubMed] [Google Scholar]
- Mukand J, Karlin L, Barrs K, Lublin P. Midodrine for the management of orthostatic hypotension in patients with spinal cord injury: a case report. Arch Phys Med Rehabil. 2001;82:694–696. doi: 10.1053/apmr.2001.22350. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ding Y, Sangha DS, Purdy RE. Upregulation of NOS by simulated microgravity, potential cause of ortho-static intolerance. J Appl Physiol. 2000;89:338–344. doi: 10.1152/jappl.2000.89.1.338. [DOI] [PubMed] [Google Scholar]
- Vaziri ND. Nitric oxide in microgravity-induced orthostatic intolerance: relevance to spinal cord injury. J Spinal Cord Med. 2003;26:5–11. doi: 10.1080/10790268.2003.11753653. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989;14:488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione per-oxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004;995:76–83. doi: 10.1016/j.brainres.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Wu W, Liuzzi FJ, Schinco FP, et al. Neuronal nitric oxide synthase is induced in spinal neurons by traumatic injury. Neuroscience. 1994;61:719–726. doi: 10.1016/0306-4522(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Kim GM, Chen S, et al. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- Kroncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PJ, Cunningham JT, Patel KP, Hasser EM. Proposed role of the paraventricular nucleus in cardiovascular deconditioning. Acta Physiol Scand. 2003;177:27–35. doi: 10.1046/j.1365-201X.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- Bayorh MA, Socci RR, Watts S, et al. L-NAME a nitric oxide synthase inhibitor, as a potential countermeasure to post-suspension hypotension in rats. Clin Exp Hypertension. 2001;23:611–622. doi: 10.1081/ceh-100107391. [DOI] [PubMed] [Google Scholar]
- Meck JV, Waters WW, Ziegler MG, et al. Mechanisms of post spaceflight orthostatic hypotension: low α1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol. 2004;286:H1486–H1495. doi: 10.1152/ajpheart.00740.2003. [DOI] [PubMed] [Google Scholar]
- Bleeker MWP, DeGroot PCE, Rongen GA, et al. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- Sanders M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sangha SD, Vaziri ND, Ding Y, Purdy RE. Vascular hyporesponsiveness in simulated microgravity: role of nitric oxide-dependent mechanisms. J Appl Physiol. 2000;88:507–517. doi: 10.1152/jappl.2000.88.2.507. [DOI] [PubMed] [Google Scholar]
- Shang-Jin S, South DA, Meck JV. Fludrocortisone does not prevent orthostatic hypotension in astronauts after space flight. Aviat Space Environ Med. 2004;75:235–239. [PubMed] [Google Scholar]
- Zhang LF. Vascular adaptations to microgravity: what have we learned? J Appl Physiol. 2001;91:2415–2430. doi: 10.1152/jappl.2001.91.6.2415. [DOI] [PubMed] [Google Scholar]
- Hargens AR, Watenpapugh DE, Breit GA. Control of circulatory function in altered gravitational fields. Physiologist. 1992;35:S80–S83. [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol. 1994;77:1776–1783. doi: 10.1152/jappl.1994.77.4.1776. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to pre-syncope in astronauts after spaceflight. J Appl Physiol. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- Broere A, Van Den Meiracker AH, Boomsam F, Derkx FHM, Man Int Veld AJ, Schalekamp ADH. Human renal and systemic hemodynamic, natriuretic, and neurohumoral responses to different doses of L-NAME. Am J Physiol. 1998;275:F870–F877. doi: 10.1152/ajprenal.1998.275.6.F870. [DOI] [PubMed] [Google Scholar]
- Sanders M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not the initiation of the hypertension induced by Nω-nitro-L-arginine-methyl-ester. Hypertension. 1997;30:64–70. doi: 10.1161/01.hyp.30.1.64. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Bohr D. The role of nitric oxide in the central control of blood pressure. Biochem Biophys Res Commun. 1995;206:77–81. doi: 10.1006/bbrc.1995.1011. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wecht JM, Bauman WA, Zeman RJ, Cardozo C. Increased iNOS expression in the thoracic aorta of SCI transected rats. J Spinal Cord Med. 2005;28:375. [Google Scholar]