Abstract
Objective:
To increase the percentage of veterans with spinal cord injuries and disorders (SCI&D) who receive annual influenza vaccinations.
Design:
A repeated measures quality improvement project using several integrated evidence-based interventions.
Setting:
23 Veterans Affairs (VA) SCI Centers.
Patients:
Veterans with SCI&D average age = 57.3 years (range 21–102 y).
Interventions:
Patient reminder letters and education; provider reminders and posters; computerized clinical reminders for vaccination targeted to SCI&D; standing orders. Main outcome measures: Patient self-reported vaccination status.
Results:
Baseline vaccination rate was 33% in fiscal year (FY) 2001. The percentage of veterans with SCI&D who reported receiving vaccinations increased from 62.5% in year 1 (FY2002) to 67.4% in FY2003 (P = 0.004); for individuals younger than 50 years of age, rates increased from 50% to 54%. Predictors of vaccination were age 65 years of age or older, VA health care visit in past year, nonsmoker, believing vaccination is important, having a health condition that may contribute to respiratory complications, and self-reported influenza in prior year.
Conclusions:
Vaccination rates were higher than baseline and higher than reported for other high-risk groups. Interventions that incorporate system-wide approaches plus patient and provider education and reminders were moderately effective in increasing vaccination rates. Targeting younger persons, smokers, and those who do not use VA care may further improve rates.
Keywords: Vaccination, Spinal cord injuries, Influenza, Prevention, Veterans, Respiratory infections
INTRODUCTION
Most persons with spinal cord injuries and disorders (SCI&D) are at high risk for respiratory complications because their respiratory muscles are typically weak and their ability to cough is impaired. These result in less effective clearing of pulmonary secretions. As a result, morbidity and mortality from respiratory-related illnesses are higher than in the general population (1,2). Data from the Model Spinal Cord Injury System indicate that the primary cause of death during the first year after injury is respiratory complications, accounting for 28% of observed deaths. Respiratory complications continue to be a leading cause of death (22%) for persons who die after the first year following SCI (2). Furthermore, persons with SCI&D who contract influenza or pneumonia are 37 times more likely to die from influenza or pneumonia complications than comparable persons from the general population (3).
Influenza vaccination is a successful method to decrease the risk of respiratory illness in persons with SCI&D. Vaccination is effective in reducing the likelihood of contracting influenza and pneumonia, lessening the severity of respiratory illnesses and decreasing the likelihood of death due to the complications of influenza or pneumonia (4–6). The effectiveness of influenza vaccination has been demonstrated in various age groups and populations (6,7), including persons with SCI&D (8).
A medical record review of documented influenza vaccinations in a sample of veterans with SCI&D followed in Veterans Health Administration (VA) SCI centers in the mid-1990s found rates of vaccination to be low (<25%) in individuals 65 years of age or older (9). In contrast, during this same period, vaccination rates in the general veteran population 65 years of age or older were significantly higher (71%). Subsequently, the VA developed a performance management system in which a set of performance indicators were used to monitor how well VA facilities were providing preventive and chronic care. The influenza vaccination measure was included as one of several indicators for both the general veteran population and veterans with SCI&D. Veterans' medical records were reviewed for the influenza vaccination season to determine how well VA facilities met targeted performance goals. By fiscal year (FY) 2001, the vaccination indicators had become performance measures for which facilities were held accountable. Despite the introduction of these performance measures, there continued to be a gap in vaccination rates between the general veteran population and veterans with SCI&D.
Earlier pilot work by the authors found that many veterans with SCI&D were not aware that they were at high risk for complications from influenza and did not know that they should receive a vaccination each year (10). We also found that some health care providers did not believe that vaccination prevented influenza, and these providers were less likely to recommend vaccination to their patients (11). Further, the ability to document details of vaccination activity (eg, patient refused, patient allergic) varied.
To develop an effective strategy to improve vaccination rates, we reviewed the literature on interventions to increase vaccination rates. Although the research did not focus on persons with SCI&D, these studies provided insights about the relative effectiveness of various interventions to improve vaccination rates. These reports used a single intervention or combined interventions at the system, patient, and/or provider level.
System Interventions
The most effective system intervention to facilitate vaccination is the use of standing orders (12–16). A standing order is a policy that directs nurses and/or pharmacists to vaccinate patients following an established protocol without an examination or specific written order from a physician. One study found that 40% of patients hospitalized in community hospitals were vaccinated against influenza in hospitals using standing orders compared with 10% of patients for whom only physician education was used (16). Similarly, Margolis (12) reported that 81% of patients were offered influenza vaccinations in an outpatient clinic that used standing orders compared with 29% of patients in a control group that did not have standing orders.
There is also strong evidence that the use of computerized clinical reminders (CCRs) is effective for increasing vaccination rates. CCRs are used to notify practitioners that a patient is due for a vaccination and allows for documentation of this activity. In one study, compliance with influenza vaccination guidelines was measured in 2 groups of internal medicine providers in an ambulatory care setting. The providers who received CCRs increased their patient vaccination rates by 78% over baseline (40% to 71%) (17). During the same period, providers who did not have CCRs had lower baseline rates and did not increase significantly from baseline (28%). Other studies have shown success in using CCRs alone (18,19). Stronger effects were seen when CCRs were used in conjunction with other interventions, such as standing orders (13).
Patient and Provider Interventions
Other interventions that focus on patients and providers have typically involved reminders and educational interventions. These interventions generally consist of phone calls, postcards, and letters that are sent to patients and/or mail reminders, newsletters, and posters sent to providers. Due to the need for annual revaccination, reminders often indicate that the influenza season is imminent and that influenza vaccinations can be received at a certain place and time. Results on the effectiveness of patient reminders for influenza vaccination have been mixed. In one study, a reminder letter was sent with educational material at the beginning of the influenza vaccination season to a random group of Medicare patients in Indiana. Follow-up reminder letters were sent to an intervention group approximately 2 months later, whereas the control group did not receive a follow-up letter. Sixty-nine percent of the intervention group was vaccinated compared with 64% in the control group (P < 0.05) (20). Buchner et al (21) found no difference in vaccination rates between a group of private outpatient clinic patients randomized to receive mailed reminders and a control group who did not receive a letter. However, Szilagyi and associates (22) demonstrated that patient reminders were effective in improving immunization rates regardless of baseline rates, patient age, setting, or vaccination type.
Several review articles have reported on the strength of using multiple interventions to increase vaccination rates. Gyorkos and associates (23) reviewed 36 studies of the effectiveness of vaccination interventions. The system-oriented interventions, that is, standing orders, demonstrated the largest effect size at 39.4% (CI: 29.7–49.0). Similarly, a review of vaccination studies by Stone and colleagues (24) found that organizational change was consistently one of the most effective interventions to increasing use of services, patient reminders were improved by being personalized, and patient education consistently had a moderate effect.
The Centers for Disease Control and Prevention (CDC) (25) published a summary of recommendations from the Task Force on Community Preventive Services about interventions to increase vaccination coverage. The following patient-oriented interventions were “strongly recommended”: client reminder/recall systems, multi-component interventions that include education, reducing out-of-pocket expenses, and expanding access in medical or public health clinical settings. For providers, the CDC strongly recommends reminders/recall and assessment/feedback about vaccinations.
The current study builds upon pilot work by the authors that used mailed reminders and educational materials targeted to veterans and their health care providers at 4 VA SCI centers (26). In that study, a sample of veterans with SCI&D was surveyed after the influenza vaccination season at the intervention sites and at 4 comparison sites, matched on number of patients treated and prior vaccination rates. Results indicated that self-reported vaccination rates were significantly higher for veterans who received mailed reminders than for those who did not receive reminders (60.5% vs 54.3%; P < 0.01) (26). Based on the literature review and our pilot work, we chose to use multiple evidence-based interventions involving patients, providers, and the VA SCI system of care for the current study. The design was essentially a repeated measures quality improvement study in which vaccination rates were monitored over time as interventions were added to improve care. We hypothesized that use of evidence-based interventions would improve vaccination rates in a high-risk population.
METHODS
Design
This 2-year repeated measures, quality improvement project (October 2002–September 2004) used 4 evidence-based interventions at 23 VA SCI centers. Historical data on influenza vaccination rates from the prior year served as our baseline (preintervention data). The 4 interventions included patient-mailed reminders and targeted education materials, reminders to vaccinate patients and educational materials given to providers, computerized clinical reminders for vaccination, and nurse standing orders. The interventions were implemented in a somewhat fluid fashion, as we made modifications during the course of the study as we learned more about what needed to be addressed to facilitate implementation of these strategies. Computerized reminders for vaccination were already available, and standing orders were already in place at some sites at the start of the study. Therefore, during the first year of the study, primary efforts were spent on the patient and provider interventions. In the second year, we expanded our efforts to system interventions, including modification of the existing vaccination CCRs to include SCI&D and an increased emphasize on the use of standing orders, as we learned about the limitations of these strategies during the first year. A random sample of veterans with SCI&D was surveyed in both years regarding their influenza vaccination status. Semistructured telephone interviews were conducted with key personnel at each site to learn what interventions were being utilized each year.
Formal Evaluation and Facilitation
A formal evaluation was conducted to identify barriers and facilitators to the interventions (27). Facilitation was used to address the barriers and assist site personnel at the SCI centers to understand and implement the suggested interventions (28). These activities included coordination of conference calls with site personnel to discuss barriers to vaccination and solicit solutions from participants, the sharing of educational information through e-mails and newsletters, and participation in monthly calls with the chiefs of the VA SCI centers.
Implementation Interventions
The ultimate goal of this project was to improve the quality of care for veterans with SCI&D. Therefore, we utilized a package of evidence-based interventions identified from the literature. In year 1, letters were mailed to all veterans with SCI&D listed on the site registries from any of the 23 VA SCI centers (N = 10,907), reminding them of the upcoming influenza vaccination season. (The list was the result of excluding deceased patients, duplicative records, veterans who were not SCI&D or had multiple sclerosis, and any missing or invalid addresses). These letters were signed by the local SCI providers and printed on the local SCI Center's stationary (ie, personalized). The content emphasized the importance of vaccination for persons with SCI&D due to their high risk for respiratory complications and specified the time and place where the veteran could obtain a vaccination. Veterans were encouraged to get vaccinated in their local communities if that was more convenient. Also enclosed in the envelope was a 1-page educational flyer that was modified from the CDC website to emphasize the high-risk characteristics of veterans with SCI&D.
Also in year 1, we prepared a letter for the SCI center service chiefs to sign and distribute to their providers. These letters called attention to the upcoming vaccination season and the importance of vaccinating patients with SCI&D due to their high-risk status. Two sets of posters were also sent to each site. The first poster encouraged patients with SCI&D to ask providers for an influenza vaccination. We asked that this poster be placed in the patient waiting areas. The other poster encouraged providers to offer their patients the influenza vaccine and to document vaccination activities in the patient medical record. We recommended that this second poster be placed at the SCI nursing station.
As part of our ongoing formal evaluation, we learned in year 1 that although the CCR for influenza was used at all of our centers as both a reminder to vaccinate patients and a way to document vaccination activity, the CCR did not specifically target veterans with SCI&D. We enlisted the assistance of information technology specialists within VA to modify the taxonomy to include all veterans with SCI&D, not just those who met standard CDC criteria (eg, >50 y of age). The project staff provided a list of diagnostic codes to be added to the taxonomy to identify persons who should be offered the vaccine. This modified taxonomy was circulated to all VA facilities so that it could be implemented in the computerized reminder system prior to year 2 of the study.
During year 2, mailed reminder letters and educational materials were sent to patients again. However, 21 of the 23 local SCI centers assumed responsibility for putting together and mailing the materials to the veterans. We emphasized that the 23 sites work with their local computer staff to ensure that the modified CCRs were installed by the start of the year 2 vaccination season. Also during year 2 we emphasized the use of standing orders for influenza vaccination at all SCI centers. Because use of standing orders is a local policy decision, we did not have direct control over this intervention and could only monitor its use during the study period. Finally, we developed additional patient educational materials. After analyzing the principal concerns raised by patients during the first year and during our pilot work (10), a second educational flyer was developed to specifically respond to these issues. For example, many patients believed that they could get “the flu” from getting vaccinated. In the second flyer, we listed the most common issues raised by patients and addressed them. In the example above, we went on to explain that the vaccine is made from a killed virus and thus it could not give them “the flu.” This second flyer was designed to accompany the first flyer, which was not modified. This new information was made available to SCI centers for use with their patients in addition to the materials previously provided.
Patient Sample
Veterans with SCI&D were identified from the local registries maintained by each SCI center. The revised survey mailing list, updated from the returned mail that resulted from the first set of reminder letters sent, contained 9,726 veterans with SCI&D. A random sample of veterans was drawn from the updated list of each SCI center for survey distribution. The sample represented 31% of the veterans from each center (total N = 3,015).
Because we were interested in change over time as a result of adding intervention components, we planned to survey the same veterans both years. However, due to the large number of veterans we were unable to locate and those who died prior to the year 1 survey mailing, we not only resurveyed all survey respondents from year 1 (n =1,733), we also randomly selected an additional 25% of veterans per SCI center in year 2 (n = 1305). These additional subjects included a sample of both nonrespondents to the year 1 survey and those who were not surveyed in the first year to maintain a comparable survey pool and response rate for both years (n = 3038; year 2).
Patient Surveys. Primary data collection involved mailed surveys for 2 consecutive years sent to a sample of veterans with SCI&D. The 3-page survey included questions on whether or not the respondent received an influenza vaccination in the past year and why and questions regarding knowledge, attitudes, and intentions towards influenza vaccination, including importance of the influenza vaccine. In addition, questions were included to self-identify comorbid health conditions that increase risk of complications (eg, diabetes, heart disease, chronic respiratory disease, chronic renal disease), risk behaviors (eg, smoking), and demographics (eg, gender, age, education, race, living situation) that could modify behavior and outcomes. Veterans were provided with an addressed, stamped envelope in which to return their completed surveys.
If veterans could not respond by mail, we asked them to contact us using a toll-free telephone number to complete the interview. If a veteran did not return a survey within 4 to 6 weeks, we contacted the veteran by telephone and if s/he agreed, we completed the interview over the telephone.
Additional Patient Data. Patient information was supplemented with data from the VA's national administrative databases. The National Patient Care Databases contain demographic, health care use, and diagnostic information. Patient age was obtained from the administrative data, and race was obtained from the administrative data if they were missing on the patient survey. The VA national Spinal Cord Dysfunction Registry database was used to obtain information about patients' level of injury. When level of injury was missing in the registry, we used information from the patient survey to determine injury level.
Analyses and Measures
To learn which patient characteristics and aspects of the study interventions were predictive of influenza vaccine receipt over the 2-year study period, we used a multilevel repeated measures logistic regression model that included random effects for the SCI center and for the veteran. We included the random effect for center to control for cluster effects that may have occurred at the SCI centers, while the individual random effect was used to control for the potential correlation of a veteran's vaccination status from year 1 to year 2 (29). Veterans could be included in one year and not another as long as their data for the available year were complete. All data analyses were performed using Stata 8.0 (StataCorp, College Station, TX).
The criterion variable for the regression model was self-reported receipt or nonreceipt of the influenza vaccination. Several veteran and SCI center characteristics were included in the model as predictor variables. Veteran characteristics included race (white vs nonwhite), age (<50 y, 50–64 y, 65+ y), college graduate (yes, no), at least part-time employment (yes, no), level of injury (paraplegia, tetraplegia), and current smoking status (smoke now: yes, no). Health conditions that may indicate a higher risk for influenza complications, including lung, heart, kidney, and immune system problems, were categorized as dichotomous yes/no responses. If veterans indicated that they had one or more of these conditions on the survey, the health conditions variable was coded as equal to 1. Variables that indicated whether or not the veteran had seen a VA health care provider in the last year (yes, no) or had self-reported having had an influenza-like illness in the past 12 months (yes, no) also were included in the model. An ordinal variable that measured the importance of the influenza vaccine on a scale from 1 to 5 was also included.
Two variables that measured the use of system level interventions by the end of year 2 were also included. Each center received a score for their use of CCR for vaccination and for presence of standing orders. If an SCI center reported using the CCR for influenza vaccination for both inpatients and outpatients, the site CCR score equaled 1. If they did not meet this criterion or if the information was missing (n = 6), they were assigned a 0. If a facility had standing orders policies in place for inpatient and outpatient settings, the standing orders variable score was 1. Facilities in which standing orders were used in only one or neither setting received a score of 0. (Because there was so little variability between sites with standing orders in both settings vs one or neither setting, this variable was dichotomized to standing orders in both settings vs one or none. Descriptive comparisons indicate that vaccination rates were not influenced by the extent to which standing orders were in place at the site). Finally, a variable indicating the study year (year 1 vs year 2) was included. The study was reviewed and approved by the institutional review board of the Hines/North Chicago VA and the University of Washington Human Subjects Division.
RESULTS
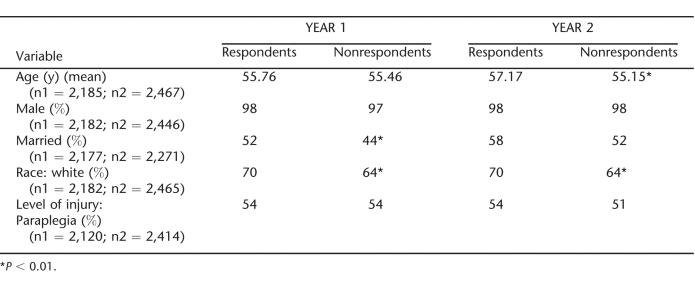
The response rates to the patient survey were 72% and 73% in years 1 and 2, respectively. Table 1 provides a comparison of respondents and nonrespondents for each year. In both years, respondents were significantly more likely to be white (χ2 = 8.25, P = 0.004; χ2 = 6.88, P = 0.009) and more likely to be married (χ2 = 12.52, P = 0.000; χ2 = 5.60, P = 0.018) than nonrespondents. In year 2, respondents were significantly older than nonrespondents (t = −3.58, P = 0.001). Respondents and nonrespondents did not differ by level of injury or gender in either year.
Table 1.
Respondents and Nonrespondents to Vaccine Survey in Years 1 and 2
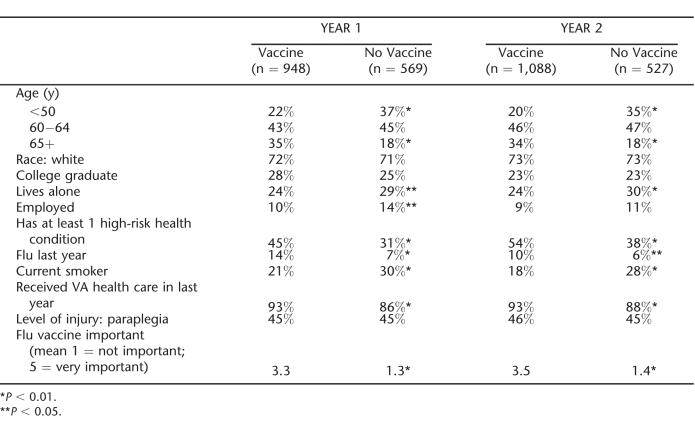
Respondents who did not have complete survey data for the variables included in the regression were excluded from analysis. A total of 1,517 patients had complete year 1 data and 1,615 had complete year 2 data. The analysis included 2,214 unique veterans; 922 (42%) had data for both years. The characteristics of the sample by vaccination status for years 1 and 2 are shown in Table 2. Self-reported vaccination rates for the entire sample increased significantly from 62.5% in year 1 to 67.4% in year 2 (χ2 =8.50, P =0.004). Vaccination rates improved for all age groups, but the increase was statistically significant for those 50 to 64 years of age. Rates for respondents younger than 50 years of age increased from 50% to 54%, from 61% to 67% in veterans 50 to 64 years of age, and from 76% to 80% in veterans who were 65 years of age or older. Chi-square tests indicated that respondents who reported receiving an influenza vaccine were more likely to: be older (year 1: χ2 = 63.19, P = 0.000; year 2: χ2 =67.42, P =0.000), not live alone (year 1: χ2 =5.21, P =0.022; year 2: χ2 =7.50, P =0.006), not be employed (year 1: χ2 = 5.088, P = 0.024), report having had the flu in the past year (year 1: χ2 =17.333, P = 0.000; year 2: χ2 = 5.22, P = 0.022), have received health care from VA in the past year (year 1: χ2 =16.15, P = 0.000; year 2: χ2 = 10.96, P = 0.001), have a health condition in addition to SCI&D that places them at higher risk for respiratory complications (year 1: χ2 = 31.62, P = 0.000; year 2: χ2 = 35.59, P = 0.000), be a nonsmoker (year 1: χ2 = 13.75, P = 0.000; year 2: χ2 = 20.71, P =0.000), and believe that receiving an influenza vaccination is important (year 1: χ2 = 699.85, P = 0.000; year 2: χ2 = 832.82, P = 0.000).
Table 2.
Subject Characteristics by Vaccination Status and by Year
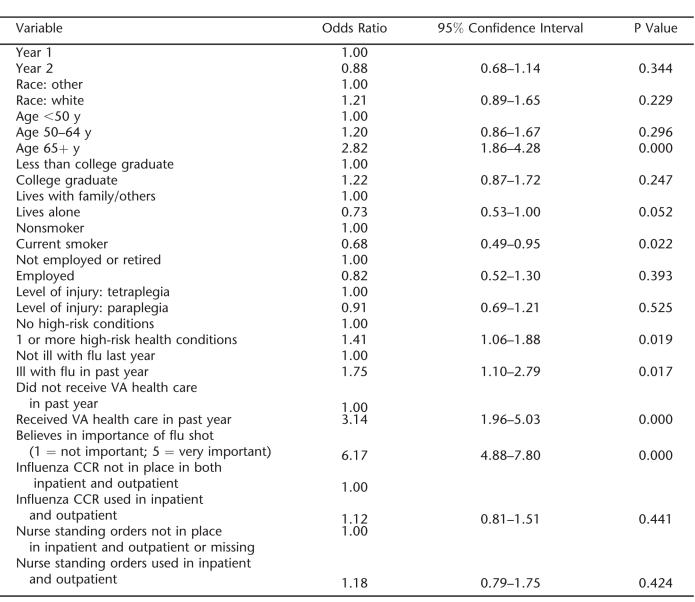
The multilevel analysis model indicated that the significant predictors of influenza vaccine receipt were patient factors including being 65 years of age or older, not being a current smoker, having self-reported influenza illness in the prior year, having received health care at the VA the past year, and holding a belief that the influenza vaccine is important (Table 3). Neither of the year 2 system-level variables was a significant predictor of vaccination. Inclusion of a random effect variable to account for the repeated measures for some individuals (those who responded to the survey both years) (29) significantly improved the fit of the model according to a log-likelihood test. However, when a random effect also was included to account for the site where the subject was assigned, this did not improve the fit of the model.
Table 3.
Multilevel Model Results of Vaccine Receipt
DISCUSSION
Historical information from VA's Office of Quality and Performance indicated that the percentage of veterans with SCI&D 65 years of age or older who received yearly influenza vaccinations was very low (≤28% through FY2000), with only slight improvement to 33% in the year prior to the start of our interventions (FY2001) (30). Research on the effectiveness of vaccination has documented its value in preventing or lessening severity of influenza symptoms and complications in the general population and in persons with SCI&D. Consequently, the purpose of this work was quality improvement, and the use of multiple interventions and implementation efforts directed at all veterans with SCI&D was warranted.
Use of multiple interventions that targeted patients, practitioners, and system changes were followed by higher vaccination rates. After the first year of interventions, the self-reported rate was 62.5% and improved by about 5% between years 1 and 2. Most veterans with SCI&D received mailed reminders and education materials in both years. Although CCRs for vaccination were available prior to our project, the reminders did not target veterans with SCI&D who were younger than 65 years of age. We also identified a number of local variations (eg, who can use the reminder, which veterans whom it is applied). Much of our facilitation work was spent working with SCI center personnel as they sought modifications of the local CCR installation to target all veterans with SCI&D and allow access to the CCR by nursing personnel who administer the vaccine (31).
Vaccination rates improved significantly for all age groups. In particular, the vaccination rates for those younger than 50 years of age and for veterans between 50 and 65 years of age were higher than those reported for other high-risk populations, such as those with diabetes (32), and increased over time. In comparison, vaccination rates for younger persons in other high-risk populations have been reported to be much lower. For example, approximately 56% of persons with diabetes who were 50 to 64 years of age and 37.8% of those 18 to 49 years of age were vaccinated in 2002 (32). Our rates were 11% and 16% higher, respectively (67% and 54%). It appears that our education efforts targeted to younger veterans with SCI&D about the serious risks of influenza and the need for vaccination, as well as changes to the vaccine reminder to include all persons with SCI&D, regardless of age, were effective in improving vaccination rates in these groups. Furthermore, by year 2, more than 80% of our veterans 65 years of age or older said that they had been vaccinated, which was higher than the reported rate for the general veteran population in the same year (70%) (30). It appears that veterans with SCI&D and their providers have recognized the importance of annual immunization as a means to reduce respiratory illness and complications.
Standing orders were in place at some facilities with SCI centers prior to our study, and additional centers adopted standing orders during our study. It was very difficult to clarify whether the policy at the facility was being fully implemented at the SCI center level. However, neither of these were significant predictors of vaccination once we controlled for patient characteristics. When combined with the multilevel modeling results, these results suggest that differences within a site (eg, case mix, age of veterans) may account for more of the variation in vaccine rates then differences between centers. Because we considered this a quality improvement effort, we had not planned to measure the relative effects of each of our interventions; rather, we considered them all part of a single intervention package to improve vaccination rates. This type of multilevel strategy is consistent with the chronic care model of care advocated by Wagner and colleagues (33,34) and is advocated by the CDC (25).
The best predictors of influenza vaccination were patient factors. Patient age continues to be a strong predictor of vaccination behavior. Veterans with SCI&D who indicated that they were current smokers were less likely to have been vaccinated. This has been found in other high-risk and older age populations as well (35,36). Efforts to improve vaccination in this group will likely require further study of attitudes, barriers, and facilitators toward vaccination in individuals who smoke.
Two illness variables, having one or more health conditions and reporting having had an influenza illness in the prior year, were predictors of vaccine receipt in our sample. The health conditions identified by our respondents (eg, heart or lung problems) are ones that are considered high risk for influenza complications and are targeted for vaccination using CDC guidelines.
Veterans who indicated that they had received health care at the VA in the past year were 3 times more likely to have been vaccinated than those who did not indicate receipt of health care from the VA. The VA has placed particular emphasis on providing influenza vaccinations to all high-risk veterans and has developed a performance measure and a national CCR for influenza vaccination. Vaccination activity is monitored through specific performance targets for influenza vaccination, and VA managers and leaders are evaluated, in part, based on how well they are able to meet or exceed these targets. Veterans who receive health care at the VA may be much more likely to be exposed to preventive care, including vaccinations, because of the large number of CCRs related to preventive care that have been implemented nationally. Other studies of influenza vaccination also have found that the presence of a primary care provider and discussion of influenza vaccination with a health care provider were significant predictors of vaccine receipt (35,37). Finally, belief in the importance of being vaccinated was strongly related to vaccine receipt (odds ratio=6.15). Positive attitudes toward the influenza vaccination have been found to be a significant predictor of vaccine receipt in other populations as well (38).
Of note, a study by Armstrong et al (39) found that individuals who received an educational brochure about influenza vaccination were significantly more likely to have reported vaccination receipt during the previous vaccination season and to indicate more interest in vaccination for the upcoming season than were those persons who only received a postcard reminder in the mail. Similarly, Moran et al (40) found that an educational brochure was more effective in increasing vaccination rates then either a financial incentive or a combination of financial incentive plus education (40). Our education plus mailed reminder intervention fits this description.
The CCR for influenza vaccination was already in place in VA medical facilities at the start of the project, so we were able to build upon this existing resource. However, as this CCR is upgraded, training for all staff should follow, or decreased effectiveness of the CCRs as training lags may follow. As new SCI staff join VA SCI centers, use of the CCRs should be included in their training. Finally, once standing orders are in place, no additional cost would be anticipated. As with CCRs, any changes in standing orders policies and training for new staff must be maintained for maximum success. Overall, the intervention package of 4 interventions used in this project represents a sustainable, broad-based method of addressing vaccination rates in our population. These intervention strategies are equally relevant and executable in non-VA settings. Standing orders exist in many settings already. In addition, the rapid move to electronic medical records in the private sector should allow adoption of some type of computerized reminder system for important screening and such preventive care activities as vaccinations.
There are several limitations to this study. Vaccine status and comorbidities are based on patient self-report. Self-reported vaccination rates in our sample were higher than what have been reported when medical records for this population are reviewed. For example, in FY2003, 68% of our respondents reported that they had received an influenza vaccination, while the Office of Quality and Performance reported that 61% of this population was vaccinated (30). It is not clear whether patients over-report or documentation is missing in the medical record. We did not have a comparison group that was not exposed to the interventions, so the effect of historical changes, such as use of performance measures for vaccination, could not be determined. However, given the high rates of morbidity and mortality in this population and the evidence regarding the effectiveness of vaccinations, not attempting to improve respiratory vaccinations at all centers could have raised ethical concerns regarding care provision. We are limited to prior year data as a proxy baseline data point. It is likely that the combination of performance measures and the interventions used in this study resulted in higher vaccination rates than either one would have produced alone. Further, the interventions used in this study were administered as a package, and we were unable to discern whether all elements of the intervention or just certain elements would have been sufficient to increase vaccination rates. Because the primary focus of this work was quality improvement, our goal was to increase rates using evidence-based interventions.
Another limitation to this work includes a package of interventions that was somewhat fluid from the first to second year. Because of our focus on quality improvement, we took an approach that allowed us to strengthen our interventions in the second year. It was also our goal to develop and implement interventions that would be sustainable after the research was completed. As such, we encouraged our sites to begin to take responsibility for some of the intervention (eg, patient mailings) while the study was ongoing. Finally, the sample of patients changed in part from year 1 to year 2. Although there were some differences in the patients surveyed from year 1 to year 2, a core group of almost 1,000 patients responded to the survey in both years. We examined the data for all respondents and for only those respondents who were the same in both years, and the results were very similar.
Strengths of the project included use of evidence-based interventions, use of multiple sites, an implementation plan that included a study team facilitator who worked with personnel at each site to identify barriers and facilitators and encouraged the staff to changes, and use of multiple measures of intervention impact. These tools are portable to other health care settings as well.
There continues to be room for improvement in this population. The Healthy People 2010 target for influenza vaccination is 90% for persons 65 of age or older (41). Continued efforts to target younger veterans, smokers, and those who are not regular users of VA health care may be warranted. Interventions that would positively influence attitudes about the value of influenza vaccine and ensure that more veterans with SCI&D are exposed to VA health care during the year may be particularly useful. Most recently, the CDC has included SCIs and other neuromuscular disorders in their list of high-risk conditions for influenza vaccination (42). There is increasing recognition that yearly influenza vaccination is important in persons with SCI&D.
CONCLUSION
Use of multiple patient, provider, and system-level evidence-based interventions was followed by increased influenza vaccination rates in a sample of veterans with SCI&D, including those who were younger. The strongest predictors for vaccination were patient factors. This suggests that educating all veterans with SCI&D about their risk of developing respiratory complications if they contract influenza and about the safety and effectiveness of influenza vaccine along with empowering them to seek out vaccination is an effective way to improve preventive behavior.
Acknowledgments
We thank the providers and staff at the 23 VA SCI centers for their assistance and support of this work, the VA SCI Strategic Healthcare Group for their support, and our veterans with SCI&D who participated in this study. The views in this article represent those of the authors and do not necessarily reflect the opinions of the Department of Veterans Affairs. A portion of these findings was presented at the annual VA Health Services Research & Development meeting, Baltimore, Maryland, February 2005.
Footnotes
This work was supported by a grant from the Health Services Research & Development Program, Research & Development Service, Department of Veterans Affairs, Washington, DC (SCT 01–169).
REFERENCES
- Wilmot CB, Hall KM. The respiratory system. In: Whiteneck GG, Charlifue SW, Gerhart KA, editors. Aging With Spinal Cord Injury. New York: Demos Publications; 1993. pp. 93–104. [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- Centers for Disease Control. Public health and aging: influenza vaccination coverage among adults aged >50 years and pneumococcal vaccination coverage among adults aged >65 years—United States, 2002. MMWR Morb Mortal Wkly Rep. 2003;52(41):987–992. [PubMed] [Google Scholar]
- Advisory Committee on Immunization Practices (ACIP) Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46(RR-8):124. [PubMed] [Google Scholar]
- Nordin J, Mulloohy J, Poblete S, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York and Oregon: data from 3 health plans. J Infect Dis. 2001;184:665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- Bridges CB, Fukuda K, Cox NJ, Singleton JA. Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2001;50(RR-4):1–44. [PubMed] [Google Scholar]
- Trautner BW, Atmar RL, Hulstrom A, Darouiche RO. Inactivated influenza vaccination for people with spinal cord injury. Arch Phys Med Rehabil. 2004;85(11):1886–1889. doi: 10.1016/j.apmr.2004.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veterans Health Administration. Spinal Cord Injury Study. Performance Report. Office of Quality and Performance; Washington, DC: March 1999. [Google Scholar]
- Evans C, Legro M, Weaver FM, Goldstein B. Perceptions about influenza vaccinations among veterans with spinal cord injury. J Spinal Cord Med. 2003;26(3):204–209. doi: 10.1080/10790268.2003.11753683. [DOI] [PubMed] [Google Scholar]
- LaVela S, Legro M, Weaver FM, Smith B. Staff influenza vaccination: lessons learned? SCI Nurs. 2004;21(3):153–157. [PubMed] [Google Scholar]
- Margolis KL, Lofgren RP, Korn JE. Organizational strategies to improve influenza vaccine delivery. Arch Intern Med. 1988;148:2205–2207. [PubMed] [Google Scholar]
- Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates: nurse protocols versus clinical reminders. J Gen Intern Med. 1999;14:351–356. doi: 10.1046/j.1525-1497.1999.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Adachi N. An effective hospital-based pneumococcal immunization program. Arch Intern Med. 1986;146:327–329. [PubMed] [Google Scholar]
- Morton MR, Spruill WJ, Cooper JW. Pharmacist impact on pneumococcal vaccination rates in long-term care facilities. Am J Hosp Pharm. 1988;45:73. [PubMed] [Google Scholar]
- Crouse BJ, Nichol K, Peterson DC, Grimm MB. Hospital-based strategies for improving influenza vaccination rates. J Fam Pract. 1994;38(3):258–261. [PubMed] [Google Scholar]
- Tang PC, LaRosa MP, Newcomb C, Gorden SM. Measuring the effects of reminders for outpatient influenza immunizations at the point of clinical opportunity. J Am Med Inform Assoc. 1999;6:115–121. doi: 10.1136/jamia.1999.0060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc. 1996;3:399–409. doi: 10.1136/jamia.1996.97084513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RP, O'Malley MS, Fletcher SW, Knight BP. Prompting physicians for preventive procedures: a five-year study of manual and computer reminders. Am J Prev Med. 1990;6(3):145–152. [PubMed] [Google Scholar]
- Smith DM, Zhou X, Weinberger M, Smith F, McDonald RC. Mailed reminders for area-wide influenza immunization: a randomized controlled trial. J Am Geriatr Soc. 1999;47:1–5. doi: 10.1111/j.1532-5415.1999.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB, White RF. Influenza vaccination in community elderly: controlled trial of postcard reminders. J Am Geriatr Soc. 1987;35:755–760. doi: 10.1111/j.1532-5415.1987.tb06354.x. [DOI] [PubMed] [Google Scholar]
- Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: a review. JAMA. 2000;284(14):1820–1827. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- Gyorkos TW, Tannenbaum TN, Abrahamowicz M, et al. Evaluation of the effectiveness of immunization delivery methods. Can J Public Health. 1994;85:S14–S30. [PubMed] [Google Scholar]
- Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–651. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults. A report on recommendations from the Task Force on Community Preventive Services. MMWR Recomm Rep. 1999;48(RR-8):1–15. [PubMed] [Google Scholar]
- Weaver FM, Goldstein B, Evans C, et al. Increasing influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2003;26(3):210–218. doi: 10.1080/10790268.2003.11753684. [DOI] [PubMed] [Google Scholar]
- Stetler CB, Legro MW, Wallace CM, et al. The role of formative evaluation in implementation research. J Gen Intern Med. 2006;21:S1–S8. doi: 10.1111/j.1525-1497.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft-Malone J, Harvey G, Seers K, Kitson A, McCormack B, Titchen A. An exploration of the factors that influence the implementation of evidence into practice. J Clin Nurs. 2004;13:913–924. doi: 10.1111/j.1365-2702.2004.01007.x. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D. Random-effects probit and logistic regression models for three-level data. Biometrics. 1997;53:1527–1537. [PubMed] [Google Scholar]
- Office of Quality and Performance. Quality Performance Reports. Washington, DC: Department of Veterans Affairs; 1998–2006. [Google Scholar]
- Wallace C, Hatzakis M, Legro MW, Goldstein B. Understanding a VA preventive care clinical reminder: lessons learned. SCI Nurs. 2004;21(3):136–142. [PubMed] [Google Scholar]
- Centers for Disease Control. Influenza and pneumococcal vaccination coverage among persons aged >65 years and persons aged 18—64 years with diabetes or asthma—United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(43):1007–1012. [PubMed] [Google Scholar]
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- Weaver FM, Goldstein B, Hammond M. Improving respiratory vaccination rates in veterans with spinal cord injury/disorders: lessons learned. SCI Nurs. 2004;21(3):143–148. [PubMed] [Google Scholar]
- Nichol KL, MacDonald R, Hauge M. Factors associated with influenza and pneumococcal vaccination behavior among high-risk adults. J Gen Intern Med. 1996;11(11):673–677. doi: 10.1007/BF02600158. [DOI] [PubMed] [Google Scholar]
- Stehr-Green PA, Sprauer MA, Williams WW, Sulivan KM. Predictors of vaccination behavior among persons ages 65 years and older. Am J Public Health. 1990;80(9):1127–1129. doi: 10.2105/ajph.80.9.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaro MK, Belue R. Prevalence of influenza vaccination in a high-risk population: impact of age and race. J Ambul Care Manage. 2005;28(1):24–29. doi: 10.1097/00004479-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Chi RC, Neuzil KM. The association of sociodemographic factors and patient attitudes on influenza vaccination rates in older persons. Am J Med Sci. 2004;327(3):113–117. doi: 10.1097/00000441-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Berlin M, Schwartz JS, Propert K, Ubel PA. Educational content and the effectiveness of influenza vaccination reminders. J Gen Intern Med. 1999;14(11):695–698. doi: 10.1046/j.1525-1497.1999.11098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran WP, Nelson K, Wofford JL, Velez R, Case DL. Inceasing influenza immunization among high-risk patients: education or financial incentive? Am J Med. 1996;101(6):612–662. doi: 10.1016/S0002-9343(96)00327-0. [DOI] [PubMed] [Google Scholar]
- Healthy People 2010. Available at http://www.health.gov/healthypeople/Document?HTML/uih/uih_4.htm. Accessed August 8, 2005.
- Harper SA, Fukuda K, Uyek TM, et al. Prevention and control of influenza: recommendations and reports of the advisory committee on immunization practices (ACIP) 2005. p. 140. [PubMed]