Abstract
Background:
Difficulty with evacuation (DWE) is a major problem after spinal cord injury (SCI). Stimulation of the anal canal and lower rectum, accomplished using a gloved finger (so-called digital rectal stimulation or DRS) is often used as an adjunct to laxatives and enemas to facilitate bowel evacuation. However, the basis for the efficacy of DRS is not known. This study assessed the effect of DRS on colonic motility.
Methods:
Six subjects with SCI were studied several hours after a bowel care session. Colonic motility was assessed using a manometric catheter (affixed endoscopically to the splenic flexure) at baseline, during DRS, and after DRS. In addition, evacuation of barium oatmeal paste (with the consistency of stool and introduced into the rectum and descending colon) was assessed simultaneously using fluoroscopic techniques.
Results:
The mean number (± SEM) of peristaltic waves per minute increased from 0 at baseline to 1.9 (±0.5/min) during DRS and 1.5 (±0.3/min) during the period immediately after cessation of DRS (P < 0.05). The mean amplitude (±SEM) of the peristaltic contractions was 43.4 (±2.2) mmHg. The frequency of contractions, as well as amplitude of contractions, during or immediately after DRS was not significantly different. These manometric changes in response to DRS were accompanied by expulsion of barium oatmeal paste in every subject by the fifth DRS.
Conclusions:
DRS causes left-sided colonic activity in subjects with SCI. At least in part, an anorectal colonic reflex that results in enhanced contractions of the descending colon and rectum may contribute to bowel evacuation in individuals with SCI.
Keywords: Digital rectal stimulation, Spinal cord injury, Neurogenic bowel, Colonic motility, Anorectal colonic reflex, Paraplegia, Tetraplegia
INTRODUCTION
There are approximately 250,000 individuals with spinal cord injury (SCI) in the United States (1). Many of these individuals have gastrointestinal symptoms due to bowel dysfunction (2,3. Indeed, difficulty with evacuation (DWE) is reported in about one third of persons with SCI (2–6). An effective bowel care regimen is mandatory in order for individuals with SCI to have an acceptable quality of life. This often requires a combination of laxatives, enemas, and suppositories. In this context, stimulation of the anal canal and lower rectum (usually termed digital rectal stimulation or DRS) is frequently used as an adjunct to promote bowel evacuation. Despite its widespread use, the basis for the efficacy of DRS is not well defined.
It has been postulated that DRS may increase motility of the left side of the colon via a stimulatory anorectal reflex (3). To study this concept further, we assessed left-sided colonic motility before, during, and after DRS in subjects with SCI using solid state manometric techniques.
METHODS
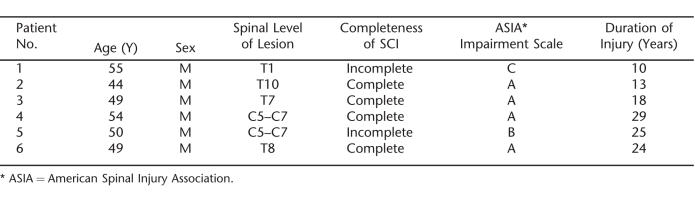
Six persons with SCI (4 with paraplegia [complete SCI in 3] and 2 with tetraplegia [complete SCI in 1]) were recruited for this study (Table 1). All subjects used DRS as part of their usual bowel care. Studies were performed during the afternoon after a subject's usual morning bowel care. Routine bowel care varied considerably between subjects and involved colonic stimulants (senna), mini-enemas, and suppositories. Informed consent was obtained from each individual before participation in the study. The study was approved by the Human Studies Subcommittee (Institutional Review Board) of the James J. Peters Veterans Affairs Medical Center.
Table 1.
Demographic Characteristics of Patients
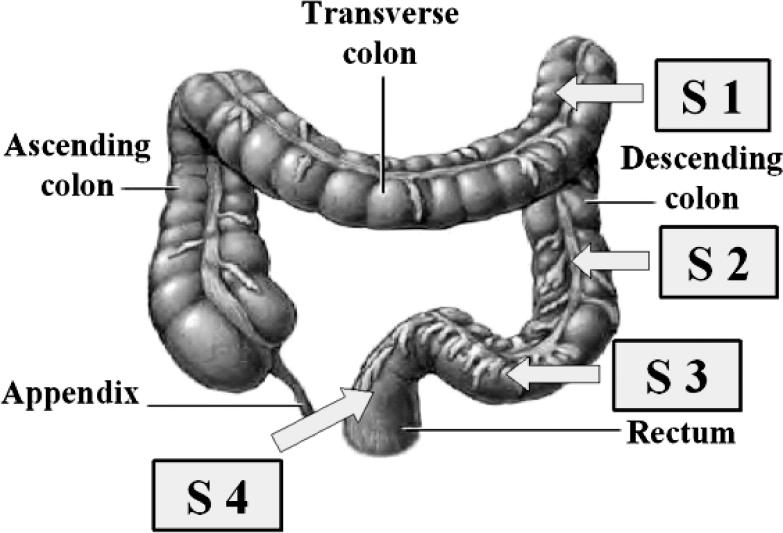
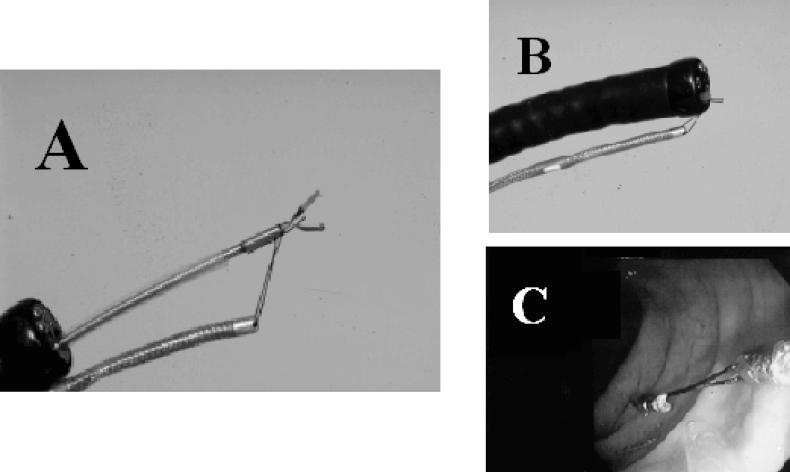
A solid state manometric probe (Gaeltec, Dunvegan, Isle of Skye, Scotland) was used to obtain manometric data from the left colon. The probe had 4 sensors separated by 10 cm (Figure 1). The proximal end of this probe was colonoscopically positioned and affixed to the splenic flexure of the colon (Figure 2A–C). The details of this procedure have been previously described (7–9).
Figure 1. Positions of the 4 sensors in the left colon (S1 = splenic flexure, S2 and S3 = descending colon, and S4 = rectum). The sensors are separated by 10 cm.
Figure 2. Technique of anchoring the manometric probe to the splenic flexure using endoclips. The probe is attached to an endoclip (Olympus, New Hyde Park, NY) using a 4-0 silk suture (A). The endoclip with attached probe is withdrawn into the end of the colonoscope (B). The colonoscope is removed from the colon after the endoclip is affixed to the splenic flexure. The probe remains tethered to the endoclip (C).
Figure 2A reprinted from Fajardo N, Hussain K, Korsten M. Gastrointestinal Endoscopy. 2000;51:199–201. With permission from the American Society for Gastrointestinal Endoscopy.
After securing the probe in place, a barium oatmeal paste (having the consistency of soft stool) was introduced into the rectum and sigmoid colon under fluoroscopic control. All subjects were assessed for (a) frequency of colonic contractions; (b) amplitude of colonic contractions; and (c) fluoroscopic evacuation of barium, as previously described by our group (10). These data were collected using a system that allowed simultaneous acquisition and storage of both manometric and fluoroscopic measurements (Kay Elemetrics, Lincoln Park, NJ). Analysis of the pressure recordings was carried out by an investigator who was unaware of the results of fluoroscopic emptying. In each subject, the frequency and amplitude of pressure spikes during DRS were compared to that which occurred before DRS (baseline) and after DRS.
After a 10-minute baseline recording, DRS was performed using a well-lubricated finger protected from radiation in a radio-opaque glove. To avoid possible allergies, latex gloves were not used (11). The gloved finger was fully inserted into the anal canal and distal rectum and stimulation delivered by gentle manipulation in a circular manner. Contact with the anal mucosa was maintained at all times. Each subject underwent digital stimulation until all the oatmeal was expelled or for a maximum of 5 repetitions. Each DRS lasted for 1 minute, with a 2-minute interval between successive DRS.
Results were expressed as mean ± SEM. Repeated measures ANOVA and Newman–Keuls tests were used for statistical evaluation.
RESULTS
Effect of DRS on the Frequency of Peristaltic Contractions
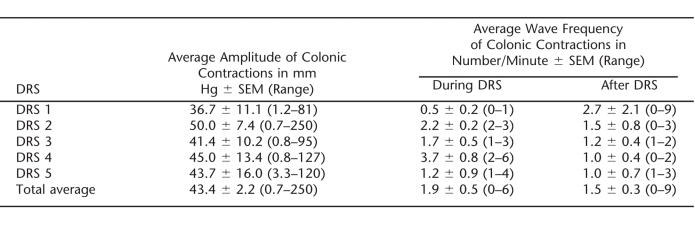
Spontaneous colonic peristaltic contractions were not observed prior to DRS during the 10-minute baseline period. During and after each DRS, peristaltic activity was noted in every subject and was captured by all 4 sensors. Compared with the baseline (0 waves/min), the mean number of peristaltic waves per minute significantly increased during DRS (1.9 ± 0.5/min, P = 0.05) and immediately after DRS (1.5 ± 0.3/min, P = 0.05) (Table 2). The frequency of contractions during DRS was not statistically different when compared with the frequency immediately after DRS. Peristaltic contractions disappeared 5 minutes after the cessation of DRS.
Table 2.
Effect of Successive Digital Rectal Stimulation (DRS) on the Contractions of the Colon
Effect of DRS on the Amplitude of Peristaltic Contractions
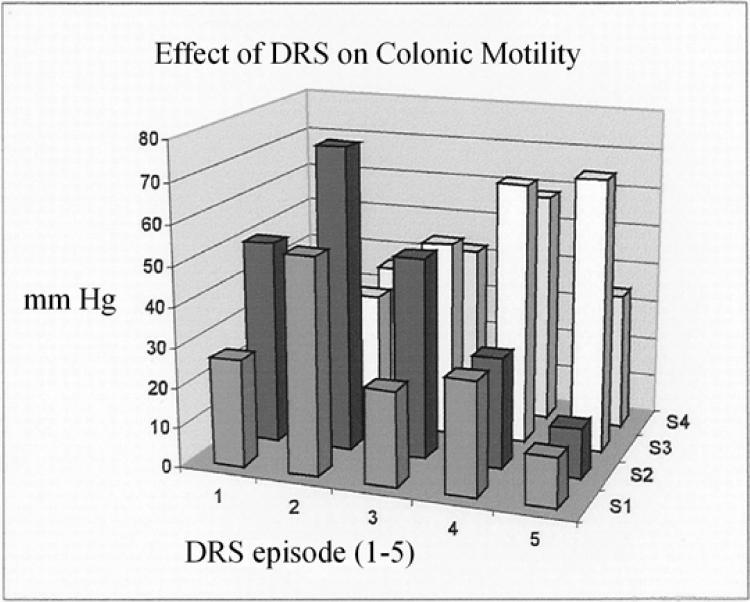
The amplitude of contractions in the left colon at the 4 different sensors during and after each DRS is shown in Table 2. Higher amplitudes were observed in the proximal rectum during the initial stimulations. During later stimulations, the highest contractile amplitudes were measured in the distal rectum (Figure 3). The average amplitude of all the peristaltic contractions of all the 5 stimulations was 43.4 ± 2.2 mmHg (range = 0.7–250 mmHg). This parameter was not significantly different when comparing contractions during and after DRS.
Figure 3. Effect of DRS (5 stimulations) on the mean amplitudes of colonic contractions at the 4 sensor sites (S1-S4).
Effect of DRS on Colonic Motility
The peristaltic contractions in the left colon were accompanied by evidence of increased motility of the left colon. This was shown as movement of the oatmeal paste on fluoroscopy. Expulsion of the barium oatmeal paste was confirmed fluoroscopically by the third DRS (7-min duration) in 1 subject, by the fourth DRS (10-min duration) in 3 subjects, and by the fifth DRS (13-min duration) in 2 subjects.
DISCUSSION
It is well established that DRS facilitates bowel movement in subjects with SCI. In part, this technique dilates the anal canal and relaxes the puborectalis muscle. Relaxation of the puborectalis decreases the anorectal angle and reduces outflow resistance to the passage of stool. The results of our present study suggest that DRS also results in contractile activity in the descending and sigmoid colon in individuals with SCI. The existence of this “ano-colonic” reflex is consistent with earlier observations made by Shafik et al (12).
Shafik et al carried out their study in 18 healthy subjects and 9 patients with SCI (T4–T6). The authors showed that rectal pressures rose in response to anal dilatation in both able-bodied volunteers and subjects with SCI. The response could be abolished by phentol-amine injection (paralyzing the internal anal sphincter) but not after blocking the pudendal nerve (the innervation of the external anal sphincter). The rectal pressure response was absent in both groups (able-bodied and SCI) after anesthetizing the rectum and anal canal. The authors concluded that anal dilatation induced by balloon distension evoked an “anorectal excitatory reflex” in subjects with SCI by stimulation of intact mechanoreceptors in the internal anal sphincter. This reflex was suspected of being capable of augmenting bowel evacuation. However, the methodology employed by Shafik et al did not permit an assessment of evacuation or colonic peristaltic activity. In contrast, the techniques employed in this study allowed simultaneous assessment of both these variables. We demonstrate here that a stimulatory reflex arising from stimulation of the anal canal and distal rectum causes an increase in the number and amplitude of peristaltic contractions. Pari passu with an increase in left-sided colonic contractile activity, there was radiological evidence of expulsion of artificial stool.
It is likely that mechanical stretch induced by DRS stimulates mechanoreceptors in the internal anal sphincter. This sensory input is mediated via S2–S4 segments, and in turn parasympathetic output of the same segments increases the motility of the left colon. This spinal reflex activation appears to promote peristalsis and may augment the mechanisms that lead to defecation.
It is still not clear, however, whether this reflex involves the spinal cord per se or the enteric nervous system (the neural network within the wall of the colon). The latter has been shown to remain intact after SCI, particularly of the upper motor neuron (UMN) type. Mechanical stimulation of the rectum has been demonstrated to produce much stronger rectal and sigmoid contractions in UMN SCI compared with lower motor neuron (LMN) SCI (13,14. All our patients with SCI had UMN lesions. Additional studies will be needed to explore the existence and strength of this excitatory ano-colonic reflex in response to DRS in individuals with LMN lesions.
There has been recent progress regarding the pharmacological management of neurogenic bowel. In this respect, neostigmine has been shown to be promising (10,15. Because neostigmine also acts by increasing parasympathetic input to the colon, DRS and neostigmine may act synergistically to improve bowel care in persons with neurogenic bowel.
DRS should be applied gently to avoid trauma and undue stretch because such stimulation can potentially injure the rectal mucosa (16) or precipitate autonomic dysreflexia in these individuals (17). However, none of our subjects developed any problems or complications as a result of using this technique. In summary, DRS contributes to bowel evacuation in individuals with SCI in part by increasing left-sided colonic motility.
REFERENCES
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Stiens SA, Fajardo NR, Korsten MA. The gastrointestinal system after spinal cord injury. In: Lin VW, editor. Spinal Cord Medicine. New York, NY: Demos Medical Publishers; 2003. pp. 321–348. [Google Scholar]
- Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86–S102. doi: 10.1016/s0003-9993(97)90416-0. [DOI] [PubMed] [Google Scholar]
- Banwell JG, Creasey GH, Aggarwal AM, Mortimer JT. Management of the neurogenic bowel in patients with spinal cord injury. Urol Clin North Am. 1993;20:517–526. [PubMed] [Google Scholar]
- Glick ME, Meshkinpour H, Haldeman S, Hoehler F, Downey N, Bradley WE. Colonic dysfunction in patients with spinal cord injury. Gastroenterology. 1984;86:287–294. [PubMed] [Google Scholar]
- Stone JM, Nino-Murcia M, Wolfe VA, Perkash I. Chronic gastrointestinal problems in spinal cord injury patients: a prospective analysis. Am J Gastroenterol. 1990;85:1114–1119. [PubMed] [Google Scholar]
- Fajardo N, Hussain K, Korsten MA. Prolonged ambulatory colonic manometric studies using endoclips. Gastrointest Endosc. 2000;51:199–201. doi: 10.1016/s0016-5107(00)70418-4. [DOI] [PubMed] [Google Scholar]
- Fajardo NR, Pasiliao R, Modeste-Duncan R, Creasey G, Bauman WA, Korsten MA. Decreased colonic motility in persons with chronic spinal cord injury. Am J Gastroenterol. 2003;98:128–134. doi: 10.1111/j.1572-0241.2003.07157.x. [DOI] [PubMed] [Google Scholar]
- Korsten MA, Monga A, Chaparala G, et al. Digital rectal stimulation causes increased left sided colonic motility in persons with SCI. Gastroenterology. 2005;128(Suppl 2):A266. [Google Scholar]
- Korsten MA, Rosman AS, Ng A, et al. Infusion of neostigmine-glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100:1560–1565. doi: 10.1111/j.1572-0241.2005.41587.x. [DOI] [PubMed] [Google Scholar]
- Shenot P, Rivas DA, Kalman DD, Staas WE, Jr, Chancellor MB. Latex allergy manifested in urological surgery and care of adult spinal cord injured patients. Arch Phys Med Rehabil. 1994;75:1263–1265. doi: 10.1016/0003-9993(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Shafik A, El-Sibai D, Shafik I. Physiologic basis of digital rectal stimulation for bowel evacuation in patients with spinal cord injury: identification of an anorectal excitatory reflex. J Spinal Cord Med. 2000;23:270–275. doi: 10.1080/10790268.2000.11753536. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF, Spillane K. An electrophysiological study of somatic and visceral convergence in the reflex control of the external sphincters. J Physiol. 1982;328:379–387. doi: 10.1113/jphysiol.1982.sp014271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WM, MacDonagh R, Forster D, Thomas DG, Small-wood R, Read NW. Anorectal function in patients with complete spinal transection before and after sacral posterior rhizotomy. Gastroenterology. 1995;108:990–998. doi: 10.1016/0016-5085(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Rosman AS, Chaparala G, Monga A, et al. Intramuscular neostigmine/glycopyrrolate improves bowel care in patients with spinal cord injury and defecatory disorders. Gastroenterology. 2005;128:A-468. [Google Scholar]
- Wang F, Frisbie JH, Klein MA. Solitary rectal ulcer syndrome (colitis cystica profunda) in spinal cord injury patients: 3 case reports. Arch Phys Med Rehabil. 2001;82:260–261. doi: 10.1053/apmr.2001.16341. [DOI] [PubMed] [Google Scholar]
- Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1979;59:1–21. [PubMed] [Google Scholar]