Abstract
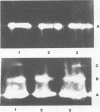
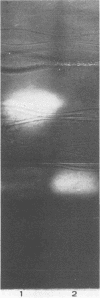
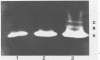
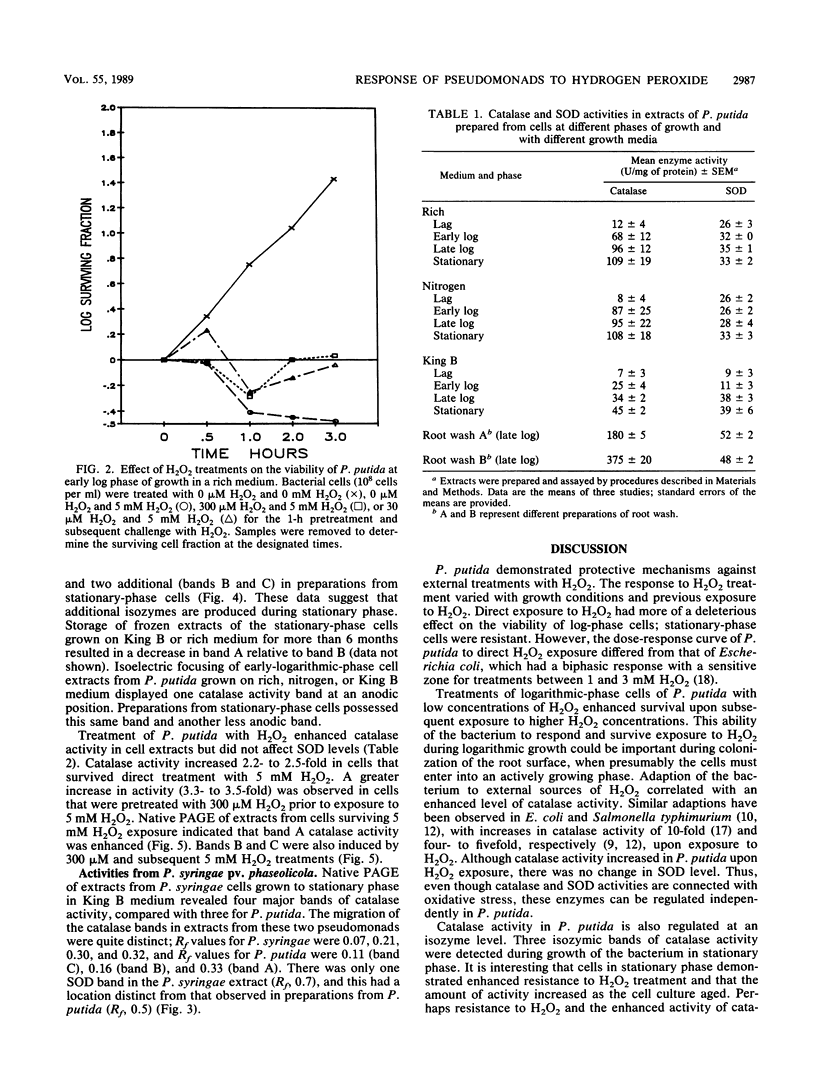
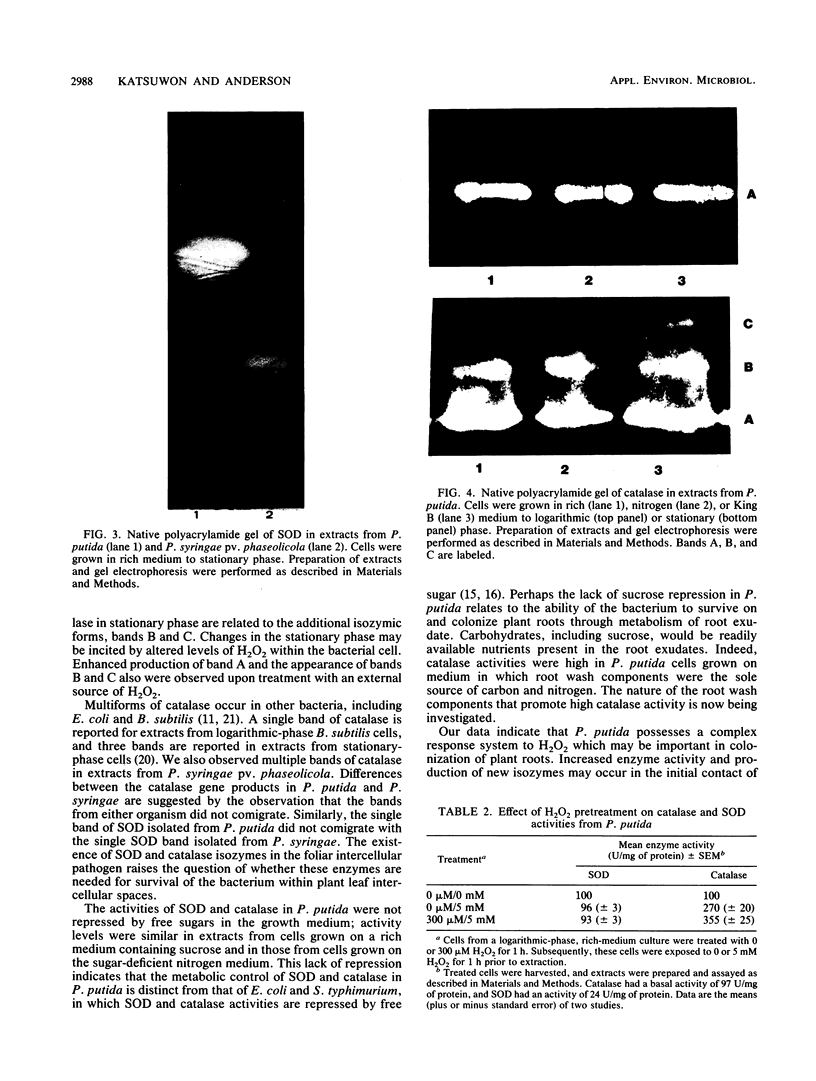
Colonization of plant root surfaces by Pseudomonas putida may require mechanisms that protect this bacterium against superoxide anion and hydrogen peroxide produced by the root. Catalase and superoxide dismutase may be important in this bacterial defense system. Stationary-phase cells of P. putida were not killed by hydrogen peroxide (H2O2) at concentrations up to 10 mM, and extracts from these cells possessed three isozymic bands (A, B, and C) of catalase activity in native polyacrylamide gel electrophoresis. Logarithmic-phase cells exposed directly to hydrogen peroxide concentrations above 1 mM were killed. Extracts of logarithmic-phase cells displayed only band A catalase activity. Protection against 5 mM H2O2 was apparent after previous exposure of the logarithmic-phase cells to nonlethal concentrations (30 to 300 μM) of H2O2. Extracts of these protected cells possessed enhanced catalase activity of band A and small amounts of bands B and C. A single form of superoxide dismutase and isoforms of catalase were apparent in extracts from a foliar intercellular pathogen, Pseudomonas syringae pv. phaseolicola. The mobilities of these P. syringae enzymes were distinct from those of enzymes in P. putida extracts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Habibzadegah-Tari P., Tepper C. S. Molecular Studies on the Role of a Root Surface Agglutinin in Adherence and Colonization by Pseudomonas putida. Appl Environ Microbiol. 1988 Feb;54(2):375–380. doi: 10.1128/aem.54.2.375-380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J. Unique aspects of the cell surface polysaccharide of Pseudomonas phaseolicola as demonstrated by bacteriophage specificity. Can J Microbiol. 1980 Dec;26(12):1422–1427. doi: 10.1139/m80-237. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Cakmak I., van de Wetering D. A., Marschner H., Bienfait H. F. Involvement of superoxide radical in extracellular ferric reduction by iron-deficient bean roots. Plant Physiol. 1987 Sep;85(1):310–314. doi: 10.1104/pp.85.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Dowds B. C., Murphy P., McConnell D. J., Devine K. M. Relationship among oxidative stress, growth cycle, and sporulation in Bacillus subtilis. J Bacteriol. 1987 Dec;169(12):5771–5775. doi: 10.1128/jb.169.12.5771-5775.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn G. J., Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):570–579. doi: 10.1128/jb.123.2.570-579.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewen P. C., Switala J. Multiple catalases in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3601–3607. doi: 10.1128/jb.169.8.3601-3607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T., Hayama E., Okuyama T. Microscale multisample two-dimensional electrophoresis of proteins in human serum, cerebrospinal fluid, and urine. Clin Chem. 1982 Apr;28(4 Pt 2):824–827. [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mottley C., Mason R. P. An electron spin resonance study of free radical intermediates in the oxidation of indole acetic acid by horseradish peroxidase. J Biol Chem. 1986 Dec 25;261(36):16860–16864. [PubMed] [Google Scholar]
- Moustafa Hassan H., Fridovich I. Regulation of superoxide dismutase synthesis in Escherichia coli: glucose effect. J Bacteriol. 1977 Nov;132(2):505–510. doi: 10.1128/jb.132.2.505-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tari P. H., Anderson A. J. Fusarium Wilt Suppression and Agglutinability of Pseudomonas putida. Appl Environ Microbiol. 1988 Aug;54(8):2037–2041. doi: 10.1128/aem.54.8.2037-2041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist L., Rannug U., Rannug A., Ramel C. Protection from toxic and mutagenic effects of H2O2 by catalase induction in Salmonella typhimurium. Mutat Res. 1984 Nov-Dec;141(3-4):145–147. doi: 10.1016/0165-7992(84)90087-3. [DOI] [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]