Abstract
Mitogen-activated protein kinases (MAPKs) phosphorylate target proteins in both the cytoplasm and nucleus, and a strong correlation exists between the subcellular localization of MAPK and resulting cellular responses. It was thought that MAPK phosphorylation was always followed by rapid nuclear translocation. However, we and others have found that MAPK phosphorylation is not always sufficient for nuclear translocation in vivo. In the developing Drosophila wing, MAPK-mediated signaling is required both for patterning and for cell proliferation, although the mechanism of this differential control is not fully understood. Here, we show that phosphorylated MAPK (pMAPK) is held in the cytoplasm in differentiating larval and pupal wing vein cells, and we show that this cytoplasmic hold is required for vein cell fate. At the same time, we show that MAPK does move into the nucleus of other wing cells where it promotes cell proliferation. We propose a novel Ras pathway bifurcation in Drosophila and our results suggest a mechanism by which MAPK phosphorylation can signal two different cellular outcomes (differentiation versus proliferation) based on the subcellular localization of MAPK.
Keywords: Drosophila, moleskin, Importin 7, Wing, MAP kinase, Cell cycle, Translocation, ERK, Ras
INTRODUCTION
The activation of MAPK is the final cytoplasmic event in the Ras signal transduction cascade, and alterations in the Ras/MAPK pathway are associated with ~25% of human tumors (Hanahan and Weinberg, 2000). MAPKs are activated by dual-phosphorylation at a specific tyrosine and threonine residue in response to many signals that control cell proliferation, growth, shape, adhesion, fate, death and other physiological responses (Perrimon, 1994; Schaeffer and Weber, 1999). These signals are often received by trans-membrane RTKs, such as EGFR, and are transduced by a cascade that includes Ras, Raf (MAPK kinase kinase) and Mek (MAPK kinase), which dually phosphorylates MAPK directly to generate phospho-MAPK (pMAPK) (reviewed by Wassarman et al., 1995). pMAPK then disassociates from MEK and forms active homodimers (Cobb and Goldsmith, 2000) that can phosphorylate targets in the cytoplasm, and/or translocate to the nucleus to activate nuclear targets, and thus controls gene expression (Brunet et al., 1999; Chen et al., 1992; Gonzalez et al., 1993; Khokhlatchev et al., 1998; Lenormand et al., 1993). Signaling specificity is achieved through several mechanisms, including restricted ligand or receptor expression, ligand processing, activating and inactivating ligands, and specific MAPK phosphatases (Rebay, 2002).
We have reported a regulated cytoplasmic sequestration of pMAPK antigen in the developing Drosophila compound eye [‘MAPK cytoplasmic hold’ (see Kumar et al., 1998)]. We developed four new reagents to detect MAPK cytoplasmic hold in vivo by a second independent method to detect nuclear MAPK (Kumar et al., 2003). All four use a heat-inducible (hsp70) promoter to drive the expression of ectopic proteins. The first is a fusion between MAPK and the yeast transcription factor GAL4 (‘MG’). When MG is expressed and translocates to the nucleus, it can specifically direct the expression of reporter genes. We also fused the SV40 nuclear localization sequence (NLS) to MG (‘NMG’), to drive the fusion protein into the nuclei of cells as a positive control. The third construct is the SV40 NLS fused to MAPK alone (no Gal4, or ‘NM’), to test the developmental consequences of forcing MAPK into cells nuclei inappropriately (i.e. breaking cytoplasmic hold). The last reagent is MAPK alone (‘M’), as a control for the simple effects of MAPK overexpression. In the developing eye, we used these reagents to detect MAPK cytoplasmic hold and show that this hold is developmentally important in the morphogenetic furrow (Kumar et al., 2003).
To address the potential role of MAPK cytoplasmic hold in the regulation of Ras/MAPK signaling in other tissues, we examined the Drosophila wing, which develops from an epithelial sheet during larval and pupal stages (Bier, 2000; de Celis, 2003). In third larval instar wing imaginal discs, pMAPK antigen is most strongly expressed in the pro-vein and wing-margin cells (Fig. 1A,B) (see Guichard et al., 1999; Martin-Blanco et al., 1999). Loss-of-function mutations in Egfr upstream functions (Rhomboid, Star, Vein) or downstream effectors [Ras, Raf and the gene encoding MAPK (rolled, rl)], each result in vein loss and, in those cases tested, loss of pMAPK antigen in larval wings (Biggs et al., 1994; Guichard et al., 1999; Martin-Blanco et al., 1999). Conversely, gain-of-function mutations in the pathway lead to increased or ectopic veins, including a dominant gain-of-function MAPK mutation, rlSem (Brunner et al., 1994; Martin-Blanco et al., 1999). In addition to patterning, wing cells lacking Ras have growth defects (Prober and Edgar, 2000). Thus, EGFR/Ras/MAPK signaling controls both cell fate (vein versus intervein) and general cell proliferation at similar times within this tissue, and the proper regulation of these two independent functions must be tightly controlled in order for the wing to develop normally.
Fig. 1. pMAPK antigen, MAPK/GAL4 (MG)-driven GFP, DNA and bs:lacZ expression in wild-type larval wings.
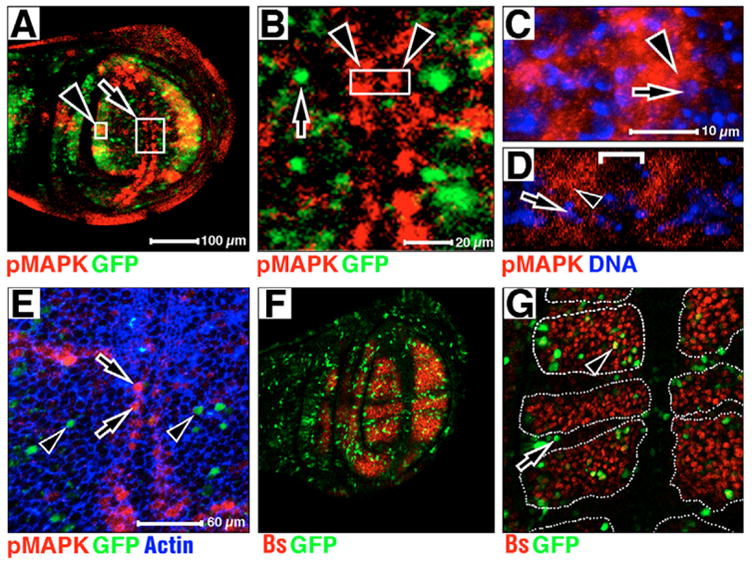
Anterior upwards, dorsal leftwards. Magnifications are equal in A and F, C and D, and E and G. (A) pMAPK is in red, MG-driven GFP is in green. (B) Confocal section of large boxed area at arrow in A. pMAPK is in red, MG-driven GFP is in green. MG-driven GFP is absent from margin cells (arrowheads). An example of a MG-driven GFP responsive nuclei is indicated by an arrow. (C,D) A single confocal section of vein tissue (C) through small boxed area at arrowhead in A, and lateral cross-section of margin tissue (D) at boxed area in B. pMAPK in red, DNA in blue. pMAPK is predominantly cytoplasmic in both regions (arrowhead indicates pMAPK staining next to pMAPK-negative nucleus at arrow). Bracket in D separates two columns of pMAPK stains at wing margin (arrowheads in B). (E) Confocal section in the wing pouch of the wing margin and veins L3, L4 and L5. pMAPK is in red, MG-driven GFP is in green, actin is in blue. Cells positive for pMAPK (arrows) and GFP (arrowheads) are indicated. (F,G) bs:lacZ is in red, MG-driven GFP is in green. GFP-positive nuclei devoid of lacZ (arrow in G), and GFP and lacZ nuclei are indicated (arrowhead in G). Broken white lines define area of bs:lacZ expression in G.
In this report, we show that pMAPK is held in the cytoplasm of developing wing vein and margin cells throughout larval and early pupal life, and that this cytoplasmic hold promotes vein differentiation. We also show that MAPK translocates to the nucleus of larval wing cells, and this nuclear translocation promotes the G1/S cell cycle transition. As Egfr is the only known RTK involved in wing development (Guichard et al., 1999), our data suggests a novel pathway bifurcation in Drosophila, where this single pathway can control both proliferation and differentiation in the developing wing through control of MAPK subcellular localization.
MATERIALS AND METHODS
Immunohistochemistry and mounting adult wings
Wing disc preparations were as described previously (Tio and Moses, 1997), mounted in Vectashield (Vector Labs, H-1000) and imaged by confocal microscopy or by bright-field microscopy with DAB and Ni/Co intensification. Primary antibodies were: mouse anti-pMAPK, Sigma (1:625, Sigma M8159) (Gabay et al., 1997), mouse anti-HSV-Tag (1:2,000, Novagen, 69171), mouse anti-Cyclin E (1:5, Iowa DBHB), rabbit anti-β-galactosidase (1:1,000, Cortex Biochem CA2190), rabbit activated caspase 3 (1:200, BD Biosciences, 551150) (Srinivasan et al., 1998), mouse anti-BrdU (1:50, BD Biosciences, 33281A), rabbit anti-Phospho Histone H3 1:1,000 (Cell Signaling Technologies 9701), rabbit anti-lamin DmO (R836, gift of Paul Fisher). Secondary antibodies were from Jackson ImmunoResearch: goat anti-mouse Cy5 (1:500, 115-175-003), goat anti-rabbit TRITC (1:250, 111-025-003), goat anti-mouse HRP (1:40, 115-035-003). DNA was stained using SYTO24 (1:10,000. Molecular Probes S-7559). Actin was detected with Rhodamine-phalloidin (1:50, Molecular Probes, A-12380). Adult wings were dehydrated in ethanol and mounted in DPX (Zeiss). Expression domain areas/cell numbers were counted using pixel counts in Photoshop.
Drosophila stocks and DNA constructs
The wild-type strain used was Canton-S. MAPK expression construct transgenics were hs:M, hs:NM, hs:MG and hs:NMG (Kumar et al., 2003); hs:rho and hs-rho30a (gifts from E. Bier) (Guichard et al., 1999); bsP1292 (a gift from M. Affolter) (Montagne et al., 1996); en:GAL4 (a gift from R. Palmer); UAS:msk and msk5 (Lorenzen et al., 2001); stg-lacZ (Lehman et al., 1999); rl10a and UAS:Argos (Freeman, 1994); rlsem (Brunner et al., 1994); UAS:p35 (FlyBase; http://flybase.bio.indiana.edu); UAS:GFP (a gift from J. Fisher); and Su(var)2055 and tkv8 (FlyBase). pBS.Kpn(RBS)MYC-DIM7 contains coding sequence of Drosophila msk (Lorenzen et al., 2001) with a N-terminal MYC epitope tag added (Hsp70-CaSpeR).
Heat shock regimes
hsp70 promoter transient expression and larval analysis
Larvae were incubated at 37°C for 1 hour, then recovered at 25°C for various times as noted in text.
Pupal analysis
Twenty-three- or 21-hour after puparium formation (APF) pupae were induced at 37°C for 1 hour and immediately dissected (0H, 23 APF) or recovered at 25°C for 2 hours (2H, 21 APF).
Adult wing analysis
Larvae were aged every 4–8 hours throughout life cycle, induced at 37°C for 1 hour, then allowed recovery at 25°C until eclosion.
Flow cytometry and adult wing analysis
hs:MG and hs:NMG larval wing discs (also containing UAS:GFP) were placed at 37°C for 1 hour, then at 25°C for 3 hours, then incubated for 3 hours in PBTH as described previously (Neufeld et al., 1998). Discs were then analyzed on DakoCytomation MoFlo. Data from at least 10,000 cells each was analyzed using FloJo software v4.5.2 (Tree Star). Adult wings of five flies from hs:M, hs:NM, hs:rho and hs:rho; hs:msk were dissected as above and wing hair counts/surface area measurements were determined as previously described (Marenda et al., 2003).
RESULTS
MAPK signaling larval wings
In late larval development, pMAPK expression is elevated in the pro-vein and wing margin cells (Fig. 1A) (Guichard et al., 1999; Martin-Blanco et al., 1999). In contrast to a previous report (Guichard et al., 1999), a more detailed analysis of the subcellular localization of pMAPK within these cells shows that the pMAPK antigen is predominately cytoplasmic during this stage (representative examples, L3 vein in Fig. 1C and margin in Fig. 1D, arrows show DNA, arrowheads show pMAPK). pMAPK is also cytoplasmic in veins L4 and L5 (data not shown). Thus, MAPK cytoplasmic hold occurs in developing larval wing cells.
If the pMAPK antigen detected in larval wings moved to the nucleus, we would expect MG-driven GFP reporter expression to mimic pMAPK expression in the wing at this time. However, MG-driven GFP expression is nearly complimentary to the cytoplasmic pMAPK antigen expression at this time (Fig. 1A–B, arrow in Fig. 1B). To quantify this, we used phalloidin to visualize individual cells and the coincidence of both pMAPK staining and MG-driven GFP reporter (Fig. 1E). In 7140 cells from five wing pouches, we found that pMAPK and GFP are coincident in 0.02% of cases. As pMAPK is only expressed in pro-vein and margin cells, our results suggest that MAPK nuclear translocation occurs predominantly in non-vein/non-margin territories. To further examine this relationship, we analyzed the coincidence of MG-driven GFP and the expression of blistered (bs:lacZ), the Drosophila Serum Response Factor, which is expressed in the majority of non-vein and non-margin cells (Fristrom et al., 1994; Montagne et al., 1996). We find that in the wing pouch, MG-driven GFP is expressed predominantly in bs:lacZ domains (Fig. 1F). Analysis of six wing pouches (Fig. 1G) showed that the majority (75–95%) of MG-driven GFP nuclei occurs in cells within bs:lacZ expression domains (as defined by outlined regions in Fig. 1G, e.g. arrowhead), although some did not (arrow in Fig. 1G). In total, 61% of the wing pouch area expresses bs:lacZ. Thus, we conclude that MAPK nuclear translocation in larval wings occurs predominantly, though not exclusively, in non-vein/non-margin cells.
MAPK signaling pupal wings
We and others observe dynamic pMAPK antigen expression in developing pupal wings (Fig. 2) (Guichard et al., 1999; Martin-Blanco et al., 1999). In early pupal development (24–27 hours APF; Fig. 2A), pMAPK expression is elevated in pro-vein and wing margin cells, while MG-driven GFP is expressed predominantly along the wing margin (arrowheads in Fig. 2A,C,D) and in two rows of cells that flank the developing veins in the distal pupal wing (arrows in Fig. 2C,D). At about 28 hours APF, we (and others) see a loss of pMAPK antigen from vein cells (compare asterisks in Fig. 2A with 2B) (Guichard et al., 1999; Martin-Blanco et al., 1999). It is possible that pMAPK is first stored in vein cell cytoplasm and becomes nuclear only at about 28 hours APF. However, we do not observe MG-driven GFP expression matching the pMAPK expression pattern detected in 24–27 APF pupal wings at, or after, 28 hours APF (out to 33 hours APF; Fig. 2A–D). It is possible that pMAPK is eliminated from the cytoplasm of pupal pro-vein cells by either dephosphorylation or proteolysis, without nuclear translocation. Again, as in larval discs, the pMAPK antigen we do detect in pupal wings is predominantly cytoplasmic (e.g. Fig. 2E,F).
Fig. 2. pMAPK antigen, MAPK/GAL4 (MG)-driven GFP and DNA expression in wild-type pupal wings.
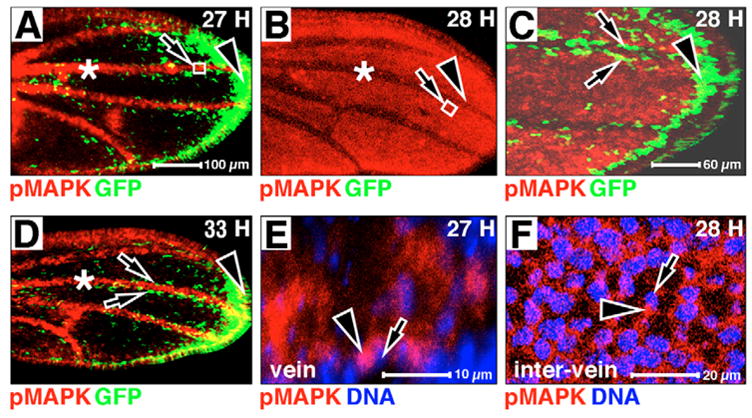
Anterior is upwards, distal is rightwards. Hours APF is indicated in the upper right-hand corner. pMAPK is in red, MG-driven GFP is in green, DNA is in blue in all panels. (A) pMAPK antigen is predominant in pupal veins (asterisk). (B) At 28 hours APF, pMAPK expression falls in the veins (compare asterisks in A and B). (C) High magnification of distal wing margin at arrowhead in B. MG-driven GFP is present in two lines of cells flanking pro-veins (arrows). (D) pMAPK returns to veins (compare asterisks in B and D). (E) High magnification of boxed area at arrow in A. (F) High magnification of boxed area at arrow in B. pMAPK is predominantly cytoplasmic in both E and F; arrowheads indicate pMAPK staining next to pMAPK-negative nuclei at arrows.
MAPK cytoplasmic hold and vein fate
Taken together, our data suggest that at the stages we observed, pMAPK antigen is predominantly cytoplasmic, and most often detected in cells in which MG-driven GFP is not. We therefore decided to test the hypothesis that the different subcellular localization of MAPK may direct different cellular outcomes in the developing wing. We have shown that we can express high levels of nuclear-directed MAPK (hs:NM) in the developing eye (Kumar et al., 2003). In order to examine the effect of increased MAPK nuclear translocation in the wing, we transiently expressed both normal (hs:M) and nuclear-directed MAPK (hs:NM) for 1 hour at various times during development. Such expression of hs:M has no affect on the pattern of veins in the adult wing (Fig. 3A). As this only increases total MAPK, and NOT activated (or pMAPK), this suggests that MAPK levels are not limiting in the wing at this time. Importantly, similar ectopic expression of hs:NM also has no effect on vein patterning (Fig. 3B), consistent with the hypothesis that nuclear MAPK does not induce vein cell fate. Similar results were obtained when both hs:M and hs:NM are raised continuously at elevated temperatures (29°C, data not shown).
Fig. 3. Overcoming MAPK cytoplasmic hold in the developing wing.
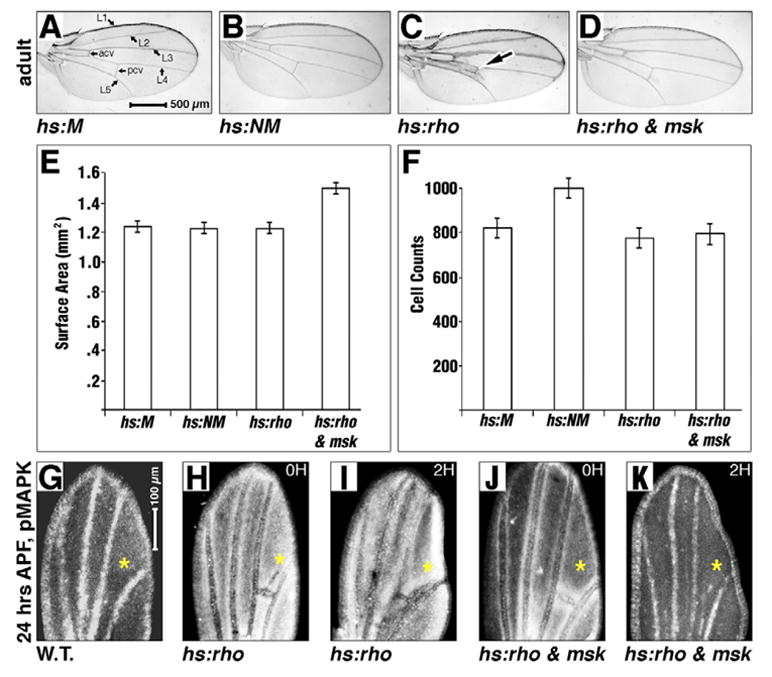
Stages are indicated on the left. Genotypes indicated. (A–D) Adult wings after a 1-hour heat induction at 20–24 APF (A,B) and 24–27 APF (C,D). (E,F) Bar graphs showing comparison of wing-surface areas (E) and cell counts/hair density (F) between genotypes depicted in A–D. The hs:rho; hs:msk wing area is larger in E; P<10−7 in each case. hs:NM wings contain more cells in F (P<10−4 in each case). (G–K) Twenty-four hour APF pupal wings (anterior leftwards, distal upwards), pMAPK antigen white. (H–K) One-hour heat induction, then recovery for the time indicated top right in hours. Magnifications are equal in A–D and G–K. Elevated pMAPK at asterisks in H and I compared with G, J and K. P values in E are: hs:M to hs:NM=0.3, hs:M to hs:rho=0.6, hs:M to hs:rho; hs:msk=4.4×10−8, hs:NM to hs:rho=0.8, hs:NM to hs:rho; hs:msk=9.1×10−9, and hs:rho to hs:rho; hs:msk=5.3×10−7. P values in F are: hs:M to hs:NM=1.3×10−4, hs:M to hs:rho=0.03, hs:M to hs:rho; hs:msk=0.1, hs:NM to hs:rho=1.0×10−5, hs:NM to hs:rho; hs:msk=1.5×10−5 and hs:rho to hs:rho; hs:msk=0.2.
Ectopic expression of hs:NM (but not hs:M) does produce blisters in 1–5% of adult wings when transiently expressed between late L3 and mid pupation (96–168 hours after egg deposition, data not shown). Further analysis of these adult wings showed that while ectopic hs:NM did not increase the overall size of the adult wing when compared with hs:M controls (Fig. 3E), it did significantly increase the number of cells within the wing when compared with hs:M (Fig. 3F) [as determined by wing hair counts in a defined area of the wing (cells per unit area, or cell density)], as each cell secretes one hair (Meyer et al., 2000). Thus, although increasing nuclear MAPK does not induce vein hypertrophy (as observed by induction of vein-promoting factors such as rhomboid, see below), it does increase cell division. We suggest that increased cell division, constrained within an unchanged surface area, can occasionally warp the epithelium, thus forming a blister.
Though expression of hs:NM causes increased cell division, it remains possible that increasing nuclear MAPK through hs:NM merely phenocopies the effect of increasing the amplitude/level of Ras signaling, as opposed to specifically increasing nuclear directed signaling downstream of receptor activation. To rule this out, we expressed the upstream EGFR activator rhomboid (rho) (Guichard et al., 1999) to increase the amplitude of pathway signaling. Overexpression of rho (hs:rho) increases pMAPK antigen in larval and pupal wings (Guichard et al., 1999). This increased pMAPK leads to vein hypertrophy (arrow in Fig. 3C) (Guichard et al., 1999), but not increased wing area or cell division (Fig. 3E,F). Thus, excess nuclear MAPK (induced by hs:NM) has a different phenotype (division) than excess cytoplasmic pMAPK induced by hs:rho (differentiation). We suggest a simple hypothesis to explain this difference: cytoplasmic pMAPK induces vein differentiation, while nuclear MAPK induces proliferation.
Increasing the amplitude of Ras signaling with hs:rho leads to increased pMAPK antigen in wings, but this antigen remains cytoplasmic, indicating that some limiting factor (downstream of pMAPK phosphorylation) is preventing the increased pMAPK generated by hs:rho from moving into the nucleus. To test whether the elevated pMAPK induced by hs:rho could induce division, we forced this ectopic pMAPK into the nucleus. Our model suggests we would expect a reduction in vein differentiation with a simultaneous increase in proliferation. To do this, we used ectopic expression of Msk: a Drosophila homolog of importin 7 (encoded by the moleskin gene, msk), which is a MAPK nuclear import cofactor, the expression of which can facilitate the nuclear translocation of pMAPK in vivo (Baker et al., 2002; Lorenzen et al., 2001). Ectopic Msk expression has been previously shown to bind to pMAPK, and to rescue loss of nuclear pMAPK in the developing Drosophila embryo (Baker et al., 2002; Lorenzen et al., 2001). We also show that ectopic expression of Msk in larval wings is sufficient to induce increased MAPK nuclear translocation (see below).
We examined the effect on pMAPK expression in pupal wings that transiently overexpress both Msk and Rho. In these wings, ectopic Rhomboid increases and alters pMAPK expression (compare asterisk in Fig. 3G with 3H), in a previously reported pattern (Guichard et al., 1999). This ectopic pMAPK persists in pupal wings for at least 2 hours after induction by hs:rho (Fig. 3I). Ectopic Msk combined with ectopic Rhomboid (hs:rho; hs:msk) suppresses the Rhomboid-induced increased pMAPK, both immediately after induction (compare asterisks in Fig. 3H with 3J) and 2 hours after induction (compare asterisks in Fig. 3I with 3K), suggesting that Msk is limiting in the developing wing. As Msk is a pMAPK nuclear import co-factor, we suggest that the addition of ectopic Msk increases the rate of nuclear translocation of pMAPK, after which nuclear phosphatase (or protease) activity eliminates the antigen. Consistent with our model, the extra veins in the adult wing induced by ectopic Rhomboid expression are strongly suppressed by the addition of ectopic Msk (Fig. 3D). In addition, hs:rho; hs:msk wings are significantly larger than either hs:rho alone (Fig. 3E), or even hs:M or hs:NM wings (Fig. 3E). Although inducing ectopic nuclear MAPK with hs:NM shows increased cell division (as evidenced by increased cell density) with no increase in organ size (suggesting that there is no coordinate increase in cell growth in these wings, and that the extra cells may be smaller than wild type cells), promoting MAPK nuclear translocation with hs:rho; hs:msk increases wing area without an increase in cell density (Fig. 3F). This suggests that there is a coordinate increase in both cell division and cell growth in these wings, and that there are now more cells of a normal size present. Thus, in both cases (hs:NM and hs:rho; hs:msk) cell division is increased. This is consistent with nuclear translocation of MAPK promoting cell division. As the Ras pathway is also known to control cell growth, our data may suggest that the signal(s) for inducing growth versus cell division by the Ras pathway split upstream of the nuclear translocation of MAPK. Importantly, increasing MAPK nuclear translocation with hs:rho; hs:msk did not broadly inhibit differentiation (inter-vein cells differentiated normally, as did wing bristles for each cell) but, rather, specifically inhibited vein cell formation, suggesting that cytoplasmic pMAPK is specific to vein cell fate (see Discussion).
Taken together, these data suggest that the elevated cytoplasmic pMAPK induced by ectopic Rhomboid directs more cells to vein fate, and that the reduction in pMAPK when we co-overexpress Msk reverses this and induces cells to divide. We suggest that the simplest interpretation is that cytoplasmic, not nuclear, pMAPK directs wing cells to vein fate, while nuclear MAPK directs cells towards proliferation.
Msk promotes MAPK nuclear translocation
The ectopic expression experiments described above are transient. To observe the phenotypic consequences of continuous and long-term reduction of MAPK cytoplasmic hold, we expressed Msk in the posterior compartment of wing discs (en:GAL4; UAS:msk or en::msk). As we are using the GAL4/UAS system to overexpress Msk in these discs, we cannot also use MAPK-GAL4 (MG) to detect MAPK nuclear translocation. Therefore, to visualize MAPK, we used HSV epitope-tagged MAPK (hs:M) and stained for the epitope tag. In control wings, the tagged MAPK is expressed at low levels, and is not specifically concentrated in any one compartment (Fig. 4A). However, when we overexpress Msk in the posterior compartment, tagged MAPK expression is visibly elevated (Fig. 4B). A closer analysis of this epitope shows that it is in many cell nuclei both where hold normally occurs (e.g. the wing margin, arrows in Fig. 4C), and also in areas where hold does not occur (data not shown), confirming that Msk overexpression increases the rate of MAPK nuclear translocation in the wing.
Fig. 4. Posterior ectopic Msk affects MAPK expression.
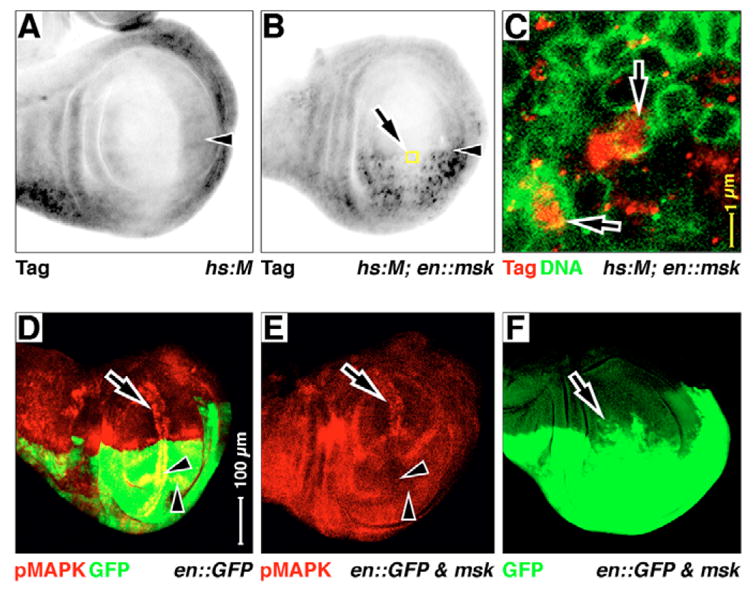
Late larval wings, anterior upwards, dorsal leftwards. Antigens and GFP are listed in the bottom left-hand corner, the expression genotype is listed in the bottom right-hand corner. (A–C) hsp70-driven expression of HSV epitope-tagged MAPK (hs:M) after a 1-hour induction. (B,C) en::msk is added to hs:M. Yellow boxed area in B is expanded in C. The epitope tag antigen is elevated where Msk is co-expressed (in the posterior, en domain in B). (C) Lamin D antigen (green) stains the nuclear envelope. Much of this MAPK is now in nuclei (arrows in C). Arrowheads in A,B indicate the AP boundary. (D–F) en::GFP (D) and en:GAL4 driving UAS:GFP and UAS:msk together (E,F). pMAPK expression that is normally associated with veins and margin (arrowheads in D) is suppressed in the posterior compartment wing pouch in E: compare non-suppressed anterior wing margin pMAPK (arrows in D,E) with suppressed posterior wing margin pMAPK and L5 pro-vein (arrowheads in D,E). The GFP expression domain expands towards the anterior (arrow in F). Magnifications are equal in A,B,D–F.
This posterior ectopic expression of Msk eliminates pMAPK antigen within pro-vein and margin cells in the posterior domain of the wing pouch (compare anterior arrows and posterior arrowheads in Fig. 4D,E). Surprisingly, posterior Msk expression disrupts the anteroposterior compartment boundary, as determined by GFP marking (arrow in Fig. 4F). High levels of cell death can disrupt development, and cause cells to cease to respect compartment boundaries. As we see high levels of cell death in Msk overexpression wings (see below), we suggest this may explain this disruption. However, even when we block cell death with p35, we still observe loss of pMAPK in the posterior wing pouch (see below), suggesting that cell death alone is not the cause of lost pMAPK in this genotype.
Taken together, these experiments suggest that ectopic Msk can increase MAPK nuclear translocation and overcome cytoplasmic hold, and that some nuclear enzyme then rapidly eliminates the pMAPK antigen (most likely a phosphatase).
MAPK nuclear translocation promotes cell proliferation
In cultured CCL39 cells, MAPK cytoplasmic tethering inhibits the ability of cells to enter S phase (Brunet et al., 1999), suggesting that MAPK nuclear translocation is important for cell cycle entry. In the developing Drosophila larval wing, elevated Ras signaling similarly promotes G1/S progression (Prober and Edgar, 2000), and MAPK loss-of-function mutations suppress this progression (Karim and Rubin, 1998). Taken together, these data suggest that the G1/S transition in the developing larval wing may require MAPK nuclear translocation. As cell proliferation in the wing is better understood in larval rather than pupal stages, we focused our analyses at this stage.
In larval wing discs, margin cells are non-proliferative [the zone of non-proliferating cells (ZNC)] (Milán et al., 1996; O’Brochta and Bryant, 1985), and markers of S-phase (BrdU) and M-phase (phospho-histone H3 antigen, pH3) are reduced in this territory (Milán et al., 1996). Similarly, MG-driven GFP is also reduced in margin territories, indicating that it too may be a marker for proliferation. However, MG-driven GFP is not in the same cells as either BrdU or pH3 (brackets in 5A,B). In the developing eye, MG-driven GFP follows the transcription of MG with a delay of 4–6 hours (Kumar et al., 2003). Thus, our observed non-coincidence of GFP with either BrdU or pH3 in the developing wing may simply be due to this time lag.
To analyze the cell cycle more precisely, we used FACS to determine the cell-cycle phase of those cells expressing MG-driven GFP, following a 1-hour induction and 6 hour recovery time (Fig. 5E). We sorted for GFP and then compared the DNA content profiles of the two cell populations (GFP− control cells with little or no MAPK nuclear translocation versus GFP+ cells where MAPK nuclear translocation has occurred). The GFP+ cell population has a slightly elevated fraction in G2 and M phase, mostly at the expense of the pool in G1 (Fig. 5E). Although these results are consistent with a function of MAPK nuclear translocation in triggering proliferation, it remains possible that MG-driven GFP is a consequence, not a cause of proliferation. To test this, we increased MAPK nuclear translocation using NMG, while simultaneously driving GFP reporter expression (hs:NMG, UAS:GFP). We induced NMG for 1 hour, followed by 6 hours recovery, and see a dramatic reduction in the fraction of GFP+ cells in G1, while greatly raising the fraction in S and G2/M (Fig. 5F), suggesting that nuclear translocation of MAPK is sufficient to induce proliferation. When we allowed these larvae to recover for 24 hours, the fraction of GFP+ cells in G2/M rose, at the expense of the pool in G1 and S (Fig. 5G). This suggests that MAPK nuclear translocation is sufficient to induce S-phase transition in wing cells, and after the initial nuclear MAPK-induced transition to S-phase, cells then progress normally through the division cycle (at least as far as G2).
Fig. 5. MAPK nuclear translocation and cell cycle in the developing wing.
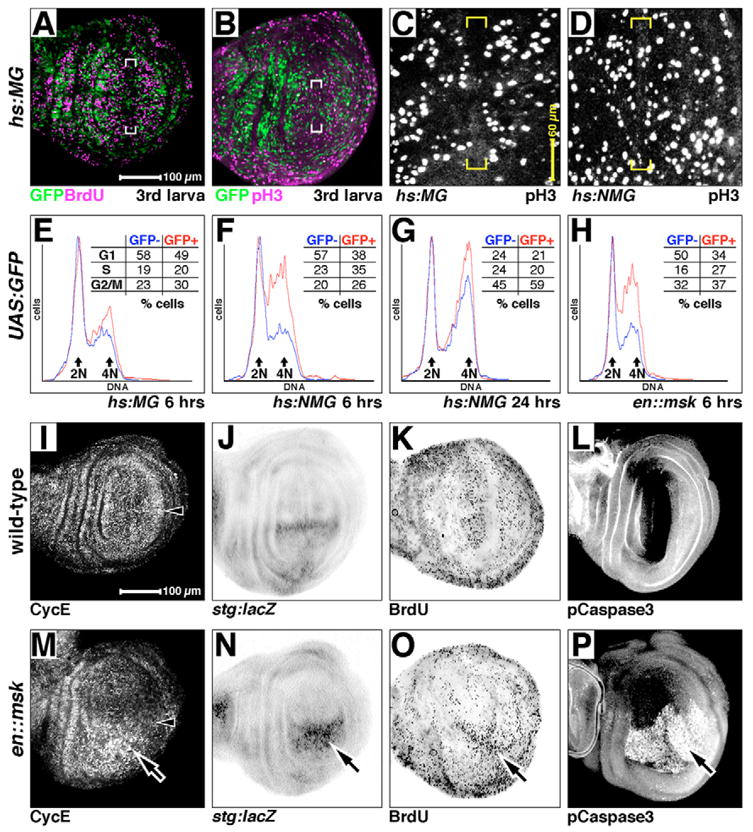
(A–D) Larval wing discs expressing hs:MG (A–C) or hs:NMG (D) after 4 hours of recovery after heat shock, showing GFP (green), bromo-deoxyuridine (BrdU, magenta in A) and phosphorylated Histone H3 (pH3, magenta in B, and white in C,D). (C,D) Confocal projections through the wing pouch of each genotype, showing each pH3-positive nuclei in the pouch. BrdU and GFP are largely restricted from non-proliferative zone in hs:MG discs (brackets in A), as are pH3 and GFP (brackets in B,C). pH3 nuclei are now observed in non-proliferative zone in hs:NMG discs (brackets in D). (E–H) Cell-sorting analysis of larval wing discs. GFP+ traces and numbers are in red, GFP− are in blue. All are from wing discs expressing GFP (UAS:GFP), driven by the genetic elements indicated. Recovery times are as indicated. DNA content on the x-axis and fraction of the cells in each sample at each DNA value on the y-axis. Major DNA content peaks (2N and 4N, corresponding with G1 and G2/M) indicated. Inset tables: percentage of cells at the stages indicated (G1, S or G2/M). (I–P) Larval discs stained for expression of cell cycle and/or apoptosis markers as indicated. (I–L) Wild type. (M–P) Ectopic Msk expressed in the posterior compartment, under the control of en:GAL4 (same domain as is green in Fig. 4D). Arrowheads in I and M indicate the AP boundary. Where Msk is ectopically expressed, Cyclin E is elevated (arrow in M), stg:lacZ is elevated (arrow in N), DNA synthesis (BrdU) is slightly elevated (arrow in O) and cell apoptosis is induced (activated Caspase 3, arrow in P). Magnifications are equal in A,B; C,D; and I–P.
However, it could be that upon induction of NMG, a block in G2/M occurs, and this allows cells to build up in S phase. To rule this out, we expressed both hs:MG and hs:NMG, and analyzed pH3 staining. We do not observe fewer pH3-positive nuclei in hs:NMG discs versus hs:MG controls (Fig. 5C,D), as would be expected if a block in G2/M existed in hs:NMG discs. Indeed, we generally see more pH3-positive nuclei in hs:NMG wing pouches when compared with hs:MG controls, along with increased pH3 staining in the ZNC (compare nuclei in brackets of Fig. 5C and 5D). These data are consistent with ectopic nuclear MAPK inducing cell proliferation, even in populations of cells that are normally non-proliferative.
We also used continuous posterior-compartment driven Msk expression (en::msk) as a second test to determine the role of MAPK nuclear translocation in wing cell proliferation. Again, the fraction of GFP+, S-phase cells is increased (27% versus 16% for the control, anterior compartment GFP− cells), as is the fraction in G2/M (37% versus 32%), at the expense of cells in G1 (Fig. 5H). As Msk is continuously available in this experiment, we interpret this as a summation of the transient 6 and 24 hour effects seen with NMG. Consistent with this, in en::msk discs we also see elevated posterior compartment expression of the S-phase limiting factor Cyclin E (compare Fig. 5I with 5M), the M-phase limiting factor String (stg:lacZ, compare Fig. 5J to 5N) and the S-phase marker BrdU (compare Fig. 5K to 5O). Taken together, these data suggest that MAPK nuclear translocation does indeed normally promote S-phase transition in developing wing cells.
Elevated proliferation in the posterior compartment might be expected to produce adult wings with enlarged posteriors (the ‘J.Lo wing’). However, prolonged and elevated expression of Msk induces caspase-dependent cell death (Fig. 5P) and the resulting adult wings are severely disrupted, with nearly normal anterior compartments and severely reduced posteriors (the ‘Twiggy wing’). These wings display loss of posterior tissue, including distal regions of veins L4 and L5, and fused posterior and anterior crossveins (en::msk in Fig. 6A) (Baker et al., 2002).
Fig. 6. Msk prevents vein fate.
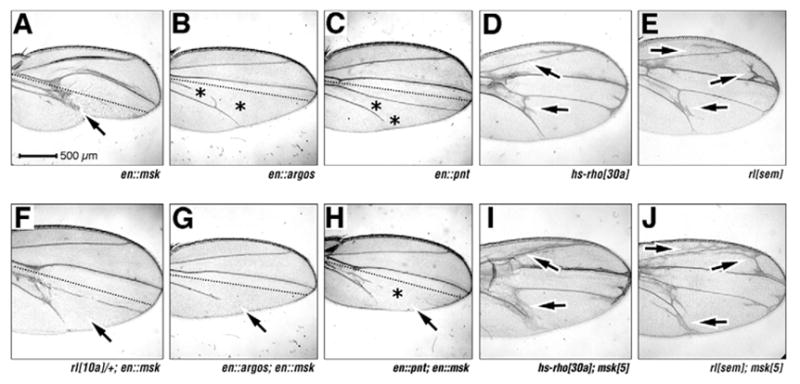
(A–J) Adult wings, anterior upwards. (A–H) Broken lines in A–C and F–H indicate the AP boundary; magnifications are equal in all panels; genotypes/driven elements are indicated. Arrows indicate Twiggy wing phenotype in A. Asterisks indicate lost veins in B,C,H. There is enhancement of vein loss by Msk (arrows in G,H) and enhanced vein formation by msk loss-of-function mutation (compare at arrows in D and I, E and J).
Msk prevents vein fate
Reduction of EGFR pathway function via loss of one copy of the gene encoding MAPK (rl10A, Fig. 6F) (Biggs et al., 1994) strongly suppresses the Msk-induced Twiggy wing, consistent with the Msk overexpression phenotype being dependant on MAPK (see Discussion). If Msk is limiting in the wing (as we suggest above), then msk gene dose should affect vein formation. msk gain-of-function should suppress vein formation, while msk loss-of-function should enhance vein formation.
To examine Msk gain-of-function, we analyzed overexpression of the negative ligand Argos in the posterior compartment of the wing, which leads to vein loss 100% in vein L4, and 90% in vein L5 (n=50, asterisks in Fig. 6B) (Freeman et al., 1992). When Msk and Argos are co-expressed, Msk enhances the vein loss phenotype of Argos to 100% in L4 and 100% in L5 (n=50, compare arrow in Fig. 6G and 6B). Similarly, overexpression of the nuclear ETS domain transcription factor Pointed P2 (PntP2, a positive MAPK effector) (Brunner et al., 1994; O’Neill et al., 1994) induces vein loss in 0% in L4 and 89% in L5 (n=54, asterisks in Fig. 6C) (Wessells et al., 1999), consistent with our suggestion that MAPK nuclear function antagonizes vein fate. Co-expression of Msk and PntP2 further enhances this vein loss to 97% in L4 and 100% in L5 (n=34, Fig. 6H).
To examine msk loss of function, we analyzed interactions of a msk null allele (msk5) with a rho gain-of-function allele (hs-rho30a, Fig. 6D), and a rolled gain-of-function allele (rlSem, Fig. 6E), which both dominantly cause extra vein formation. Trans-heterozygous hs-rho30a/msk5 wings show a strong enhancement of the rho extra-vein phenotype (compare at arrows in Fig. 6D with 6I) (see Baker et al., 2002). Similarly, trans-heterozygous rlSem/msk5 wings also show enhancement of the rolled extra vein phenotype (compare arrows in Fig. 6E with 6J). Furthermore, Msk gain of function suppresses the extra veins caused by both hs-rho30a and UAS:rlSem expression (see Fig. S1E-G in the supplementary material).
Though these effects may reflect additive genetic phenotypes as opposed to true genetic interactions, when taken together, our gain-of-function and loss-of-function data suggest that Msk normally functions to restrict vein formation. We suggest this is because gain of msk function leads to increased nuclear MAPK (vein loss), while loss of msk leads to increased cytoplasmic MAPK (extra veins). These data are consistent with our suggestion above: that vein formation through MAPK occurs through a cytoplasmic, rather than a nuclear target.
Msk vein loss is not due to cell death
As posterior overexpression of Msk leads to extensive cell death, and ultimately the Twiggy wing, vein loss could be due to tissue loss in the posterior compartment. To block any cell death associated with Msk overexpression, we co-expressed Msk with the baculovirus pan-caspase inhibitor p35. Posterior ectopic expression of p35 has no effect on the expression of pMAPK in the larval wing (compare Fig. 7A with 7C). Surprisingly, p35 expression leads to vein loss in the adult wing (asterisk in Fig. 7G). Importantly, posterior co-expression of Msk with p35 leads to a similar loss of pMAPK expression in larval wings as that observed with ectopic expression of Msk alone (compare Fig. 7D with 7B and 7A), suggesting that cell death is not the cause of lost pMAPK expression in larval wings expressing Msk in this tissue. Furthermore, the adult vein loss observed with p35 expression is significantly enhanced by co-expression with Msk (compare Fig. 7H with 7F and 7G), resulting in severely distorted wings with enlarged posterior compartments compared with the control anterior compartments (the ‘J.Lo wing’). We suggest that co-expression of p35 with Msk blocks Msk-induced cell death, thus increasing proliferation through the nuclear translocation of MAPK in the posterior compartment, but also decreasing tissue loss associated with cell death, resulting in an enlarged posterior wing.
Fig. 7. Ectopic Msk vein loss is not due to cell death.
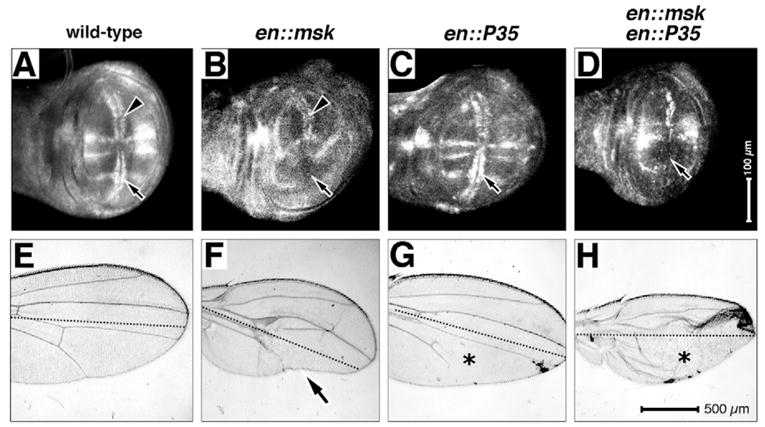
(A–D) Larval wings, anterior upwards, dorsal rightwards. Magnifications are equal in all panels, genotypes are as indicated. Arrows and arrowheads indicate pMAPK antigen expression or its loss. (E–H) Adult wings, anterior upwards, broken lines indicate the AP boundary; magnification is equal in all panels and genotypes are as indicated. Asterisks indicate lost veins in G,H. Arrow indicates the Twiggy wing phenotype in F.
DISCUSSION
Previously, we have described ‘MAPK cytoplasmic hold’ in the developing eye: the retention of pMAPK in the cytoplasm for an extended period (Kumar et al., 2003; Kumar et al., 1998). Here, we report the existence and contribution of MAPK cytoplasmic hold in the developing Drosophila wing. We observe a difference in cytoplasmic versus nuclear function of MAPK, and suggest that in the developing wing, MAPK subcellular localization controls the difference between vein specification (cytoplasmic MAPK) and proliferation (nuclear MAPK).
Cytoplasmic hold and vein fate: direct versus indirect mechanism
Perhaps vein differentiation is simply an indirect effect of repressing cell proliferation by inhibiting MAPK nuclear translocation. To address this, we analyzed vein formation in adult wings overexpressing both positive and negative cell cycle regulators (see Fig. S1 in the supplementary material). If vein formation is lost by inducing cell proliferation with effects other than forced nuclear MAPK, this would argue that the observed vein loss in en::msk is most probably due to an indirect effect of disrupting cell proliferation, as opposed to disrupting cytoplasmic pMAPK.
Overexpression of either CycE or Stg leads to increased proliferation in Drosophila wings (Neufeld et al., 1998); however, there is little to no effect on vein formation, with no vein loss in either case (see Fig. S1A,B in the supplementary material). Similarly, inhibiting cell proliferation by over-expressing either the cyclin-dependant kinase inhibitor dacapo, or the S-phase inhibitor p21 had no significant effect on vein formation (see Fig. S1C,D in the supplementary material). This is consistent with a direct effect for MAPK cytoplasmic hold on vein differentiation.
There are a number of known cytoplasmic targets of MAPK, including p90RSK, cPLA2 and Myosin light chain kinase (Ebisuya et al., 2005). However, it is important to consider that some cytoplasmic target proteins for MAPK may first be phosphorylated in the cytoplasm and then translocate to the nucleus, or be inhibited from doing so, such as SV40 T-antigen and Xenopus nucleoplasmin (Johnson et al., 2004). In fact, Hasson et al. have recently reported that the co-repressor Groucho is directly phosphorylated by MAPK, and this phosphorylation weakens its repressor activity, leading to extra veins (Hasson et al., 2005). Groucho, though it functions as a nuclear transcription factor, may be phosphorylated in the cytoplasm in pro-vein cells, where it can then translocate to the nucleus to affect changes in Notch transcription, leading to vein formation.
Mechanism of cytoplasmic hold: pMAPK anchoring vs import sequestration
Recent reports suggest that MAPK cytoplasmic hold may perform similar functions in mammals (Ebisuya et al., 2005). In vertebrate cells, expression of the death effector PEA-15 can sequester pMAPK in the cytoplasm (Formstecher et al., 2001). After treatment with Retinoic acid, embryonic stem and carcinoma cells stop proliferating, restrict the nuclear entry of pMAPK and differentiate into primitive endoderm (Smith et al., 2004). In the mouse embryo, pMAPK is detected in the cytoplasm rather than the nuclei of cells receiving FGF signals (Corson et al., 2003). A family of proteins called SEFs antagonize MAPK signaling (Fürthauer et al., 2002). More recently, SEF has been found to act directly to hold pMAPK in the cytoplasm, suggesting a mechanism for FGF pathway attenuation through MAPK cytoplasmic hold (Torii et al., 2004; Tsang and Dawid, 2004). We have been unable to identify any homolog of PEA-15 or SEF outside the chordates by conventional bioinformatic techniques. However, a fly protein with a function that is very similar to SEF would fit the MAPK cytoplasmic hold phenomena we observe in the eye and wing.
While anchoring of pMAPK has been shown to restrict MAPK nuclear entry in cell culture, it remains possible that pMAPK nuclear import could be prevented by removing a required nuclear import co-factor. Thus, by cytoplasmic sequestration of Msk (for example), pMAPK would be unable to translocate into the nucleus, and pMAPK cytoplasmic hold would be achieved.
Regardless of the mechanism, MAPK cytoplasmic hold may be a conserved mechanism necessary for the differentiation of certain developing tissues in many taxa, and proper control of MAPK subcellular localization may act as a developmental signal to determine the proliferative state of a cell.
Mammalian importin 7 is reported to import several proteins into the nucleus, including histone H1, core histones, HIV-1 reverse transcription complexes and the glucocorticoid receptor (Baake et al., 2001; Fassati et al., 2003; Freedman and Yamamoto, 2004; Jäkel et al., 1999). However, our data suggest that MAPK is a crucial target for the phenotypes we observe in wings overexpressing Msk: (1) a null mutation in Drosophila MAPK strongly suppresses the en::msk adult wing phenotype; (2) increased nuclear MAPK is observed after overexpression of Msk in larval wings; (3) loss-of-function mutations in Drosophila Histone H1 (Su(var)205) have no effect on the en::msk phenotype (data not shown); (4) loss-of-function mutations in members of other vein promoting pathways (thick veins, tkv8) have no effect on the en::msk adult wing (data not shown).
In the developing compound eye, breaking MAPK cytoplasmic hold in cells within the morphogenetic furrow results in reduced expression of Atonal, which is required for the initiation of differentiation in the developing eye (Kumar et al., 2003). Taken together with our new data from the developing wing, we suggest that MAPK cytoplasmic hold may be generally required for the cell cycle arrest necessary for the initiation of differentiation, thus defining a novel bifurcation in the Ras pathway to control different cellular outcomes. Finally, the regulation of MAPK cytoplasmic hold may help to distinguish the MAPK signals for cell fate from those for cell proliferation.
Supplementary Material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/133/1/43/DC1
Acknowledgments
We thank Marcus Affolter, Ethan Bier, Ruth Palmer, Paul Fisher, Janice Fisher and Bruce Edgar for gifts of reagents; Mike Hulsey and Ken Moberg for help with FACS analysis; and Daniela Zarnescu and Ken Moberg for reading our manuscript. D. Marenda is supported by NIH fellowship 1 F32 GM073608. Work in the Perkins’ laboratory was supported by NIH RO1 GM61707 and PO1 HD39942 Project 2, and in the Moses’ and Powers’ laboratories by NIH R01 EY12537.
References
- Baake M, Bauerle M, Doenecke D, Albig W. Core histones and linker histones are imported into the nucleus by different pathways. Eur J Cell Biol. 2001;80:669–677. doi: 10.1078/0171-9335-00208. [DOI] [PubMed] [Google Scholar]
- Baker SE, Lorenzen JA, Miller SW, Bunch TA, Jannuzi AL, Ginsberg MH, Perkins LA, Brower DL. Genetic interaction between Integrins and moleskin, a gene encoding a Drosophila homolog of Importin-7. Genetics. 2002;162:285–296. doi: 10.1093/genetics/162.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. Drawing lines in the Drosophila wing: initiation of wing vein development. Curr Opin Genet Dev. 2000;10:393–398. doi: 10.1016/s0959-437x(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Biggs WHr, Zavitz KH, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky SL. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994;13:1628–1635. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssegur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. Dimerization in MAP-kinase signaling. Trends Biochem Sci. 2000;25:7–9. doi: 10.1016/s0968-0004(99)01508-x. [DOI] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- de Celis JF. Pattern formation in the Drosophila wing: The development of the veins. BioEssays. 2003;25:443–451. doi: 10.1002/bies.10258. [DOI] [PubMed] [Google Scholar]
- Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- Fassati A, Gorlich D, Harrison I, Zaytseva L, Mingot JM. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–3685. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstecher E, Ramos JW, Fauquet M, Calderwood DA, Hsieh JC, Canton B, Nguyen XT, Barnier JV, Camonis J, Ginsberg MH, et al. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15:2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. Misexpression of the Drosophila argos gene, a secreted regulator of cell determination. Development. 1994;120:2297–2304. doi: 10.1242/dev.120.8.2297. [DOI] [PubMed] [Google Scholar]
- Freeman M, Klämbt C, Goodman CS, Rubin GM. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell. 1992;69:963–975. doi: 10.1016/0092-8674(92)90615-j. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Gotwals P, Eaton S, Kornberg TB, Sturtevant M, Bier E, Fristrom JW. blistered: a gene required for vein/intervein formation in wings of Drosophila. Development. 1994;120:2661–2671. doi: 10.1242/dev.120.9.2661. [DOI] [PubMed] [Google Scholar]
- Fürthauer M, Lin W, Ang SL, Thisse B, Thisse C. Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol. 2002;4:170–174. doi: 10.1038/ncb750. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, Howard K, Bier E. rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development. 1999;126:2663–2676. doi: 10.1242/dev.126.12.2663. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hasson P, Egoz N, Winkler C, Volohonsky G, Jia S, Dinur T, Volk T, Courey AJ, Paroush Z. EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat Genet. 2005;37:101–105. doi: 10.1038/ng1486. [DOI] [PubMed] [Google Scholar]
- Jäkel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Gorlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–2423. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. BioEssays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP Kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Hsiung F, Powers M, Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development. 2003;130:3703–3714. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman DA, Patterson B, Johnston LA, Balzer T, Britton JS, Saint R, Edgar BA. Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development. 1999;126:1793–1803. doi: 10.1242/dev.126.9.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen JA, Baker SE, Denhez F, Melnick MB, Brower DL, Perkins LA. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development. 2001;128:1403–1414. doi: 10.1242/dev.128.8.1403. [DOI] [PubMed] [Google Scholar]
- Marenda DR, Zraly CB, Feng Y, Egan S, Dingwall AK. The Drosophila SNR1 (SNF5/INI1) subunit directs essential developmental functions of the Brahma chromatin remodeling complex. Mol Cell Biol. 2003;23:289–305. doi: 10.1128/MCB.23.1.289-305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Roch F, Noll E, Baonza A, Duffy JB, Perrimon N. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development. 1999;126:5739–5747. doi: 10.1242/dev.126.24.5739. [DOI] [PubMed] [Google Scholar]
- Meyer CA, Jacobs HW, Datar SA, Du W, Edgar BA, Lehner CF. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 2000;19:4533–4542. doi: 10.1093/emboj/19.17.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milán M, Campuzano S, Garcia-Bellido A. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc Natl Acad Sci USA. 1996;93:640–645. doi: 10.1073/pnas.93.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development. 1996;122:2589–2597. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- O’Brochta DA, Bryant PJ. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature. 1985;313:138–141. doi: 10.1038/313138a0. [DOI] [PubMed] [Google Scholar]
- O’Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Perrimon N. Signaling pathways initiated by receptor protein-tyrosine kinases in Drosophila. Curr Opin Cell Biol. 1994;6:260–266. doi: 10.1016/0955-0674(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Rebay I. Keeping the receptor tyrosine kinase signaling pathway in check: lessons from Drosophila. Dev Biol. 2002;251:1–17. doi: 10.1006/dbio.2002.0806. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Smedberg JL, Rula ME, Xu XX. Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J Cell Biol. 2004;164:689–699. doi: 10.1083/jcb.200312028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Tio M, Moses K. The Drosophila TGFa homolog Spitz acts in photoreceptor recruitment in the developing retina. Development. 1997;124:343–351. doi: 10.1242/dev.124.2.343. [DOI] [PubMed] [Google Scholar]
- Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7:33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci STKE. 2004;2004:1–5. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Therrien M, Rubin GM. The Ras signaling pathway in Drosophila. Curr Opin Genet Dev. 1995;5:44–50. doi: 10.1016/s0959-437x(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Grumbling G, Donaldson T, Wang SH, Simcox A. Tissue-specific regulation of vein/EGF receptor signaling in Drosophila. Dev Biol. 1999;216:243–259. doi: 10.1006/dbio.1999.9459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/133/1/43/DC1


