Abstract
Following a bite from an infected tick, tick-borne flaviviruses cause encephalitis, meningitis and hemorrhagic fever in humans. Although these viruses spend most of their time in the tick, little is known regarding the virus-vector interactions. We developed a simple method for synchronously infecting Ixodes scapularis larvae with Langat virus (LGTV) by immersion in media containing the virus. This technique resulted in approximately 96% of ticks becoming infected. LGTV infection and replication were demonstrated by both viral antigen expression and the accumulation of viral RNA. Furthermore, ticks transmitted LGTV to 100% of the mice and maintained the virus through molting into the next life stage. This technique circumvents limitations present in the current methods by mimicking the natural route of infection and by using attenuated virus strains to infect ticks; thereby, making this technique a powerful tool to study both virus and tick determinants of replication, pathogenesis and transmission.
Keywords: tick, Ixodes scapularis, Langat virus, tick-borne encephalitis, immersion, transmission, virus replication
Introduction
Viruses transmitted via the bite of an infected tick cause infections worldwide and can produce diseases with high mortality and morbidity. One such group of viruses is the tick-borne flaviviruses which include Tick-borne encephalitis virus (TBEV), Omsk hemorrhagic fever virus (OHFV), Kyasanur forest disease virus (KFDV), Louping ill virus, Powassan virus and Langat virus (LGTV). Infection with these viruses can result in a range of clinical symptoms, from mild febrile forms to severe, sometimes fatal, meningoencephalitis and hemorrhagic fever. In addition, chronic infections associated with long term neurological sequelae have also been described (Gritsun et al., 2003). In spite of the distinct clinical syndromes, all of these viruses are closely related and the genetic basis for the differences in the pathogenicity is poorly understood. A possible connection between the arthropod vector species and pathogenicity has been proposed for the flaviviruses (Gaunt et al., 2001; Gritsun et al., 2003; Nuttall et al., 1991). Thus, a deeper understanding of the interaction between these viruses and their tick vector is warranted.
Tick-borne flaviviruses are maintained in nature through a transmission cycle involving both a vertebrate host and a tick vector. The prominent vectors for this enzootic cycle are the ixodid ticks. Once these ticks emerge from the egg, they have three parasitic stages of life (larva, nymph and adult). During these stages the tick must engorge on a vertebrate host prior to advancing to the next phase in their life cycle (Nuttall and Labuda, 2003). The ixodid tick may become infected with these viruses at any developmental stage, by either horizontal (between vertebrate host and tick), transstadial (across stages of life) or vertical (across tick generations) transmission, and the infection can be maintained throughout the tick’s lifespan (Chernesky and McLean, 1969; Costero and Grayson, 1996; Nuttall and Labuda, 2003). Since the tick’s lifespan is measured in years and since the viruses spend the majority of their time in the tick vector, it is estimated that 95% of the tick-borne flavivirus evolutionary lifespan is spent in ticks rather than in the vertebrate host (Nuttall et al., 1991; Nuttall and Labuda, 2003). As a result, ticks have an enormous influence on the persistence of tick-borne flaviviruses in nature as well as on the genotype and possible phenotype of the virus.
Despite the importance of ticks as a vector for flavivirus, little is known regarding the virus-vector interactions important for pathogen replication in the tick and transmission of the virus to a vertebrate host. The technical limitations associated with the two fundamental methods that currently exist for infecting ticks in a laboratory setting have restricted these studies. One currently used method is the feeding of ticks on a viremic vertebrate host. Although this is a natural means of infecting ticks, this method is dependent on a virus that replicates in the vertebrate host and that reaches a sufficient level of viremia for transmission to a naïve tick (Chernesky, 1969). Consequently, this technique is not suitable for studying virus-vector interactions of attenuated virus strains or strains impaired for replication in the vertebrate host system. In addition, this method does not allow for studies involving synchronously infected ticks with a defined viral inoculum. The second method for infecting ticks is via parenteral inoculation by microinjection. This method bypasses the midgut barrier, which is known to be a critical determinant for vector competency in nature. Thus, this method does not represent a natural route of infection (Jones et al., 1989; Steele and Nuttall, 1989). Furthermore, the fact that many of these viruses require high biocontainment at Biosafety Level (BSL) 3 or BSL-4 adds to the technical difficulties of working with these pathogens.
LGTV is a naturally attenuated flavivirus that can be safely worked with at BSL-2 and therefore is used as a model of the more virulent tick-borne flavivirus infections. Utilizing LGTV, we developed a simple technique of infecting ticks that circumvents the procedural restrictions present in the currently used methods. This new technique was adapted from an immersion procedure developed by Policastro and Schwan (2003). Infection by this method resulted in replication of LGTV within I. scapularis larvae. In addition, ticks infected with LGTV by this method were competent to horizontally transmit LGTV to a mammalian host and to transstadially transmit LGTV to the nymphal stage. This method of synchronously infecting ticks by immersion will be a powerful tool to study interactions between tick-borne viruses, tick vectors and vertebrate hosts.
Results
Infection of I. scapularis larvae by immersion in LGTV
To perform detailed studies of virus–vector interactions, we developed a simple method that enabled us to synchronously infect large numbers of tick larvae with a tick-borne flavivirus. Since previous studies have suggested that I. scapularis ticks might be competent for LGTV (Lawrie et al., 2004; Varma and Smith, 1972; Xu et al., 2003), we utilized a method developed for infecting I. scapularis larvae with Borrelia burgdorferi (Policastro and Schwan, 2003). Following infection, no differences in mortality were observed between LGTV infected and mock infected groups of larvae at 28 days post immersion (dpim) indicating that infection with LGTV does not lead to reduced survival of the larvae (data not shown).
Characterization of LGTV infection in I. scapularis larvae
Infection and replication of LGTV in the tick larvae were evaluated by several methods. First, dissected midgut preparations were examined for the presence of viral antigens by immunofluorescence at 17 dpim. Viral antigen was observed throughout the midgut of the ticks immersed in media containing virus, but not in ticks immersed in media alone (Fig. 1). In addition, the LGTV antigen was distributed in the cytoplasm of the infected cells, which is the primary intracellular site of flavivirus replication. These results demonstrated that LGTV antigen was present in the appropriate target organ of tick larvae following infection by immersion.
Figure 1. Viral antigen expression in the midgut cells of larval ticks.
Ticks were immersed in media alone (mock) or in media containing LGTV (infected). Midgut sections were stained for the presence of LGTV antigen (green). DAPI (blue) was used to counterstain the nuclei.
To determine the efficacy of the immersion technique to infect ticks, individual ticks were analyzed for the presence of LGTV RNA. Using RT-PCR, we could readily detect LGTV RNA at 15 dpim (data not shown). Hence, this timepoint was selected to determine the efficiency of LGTV infection in ticks by immersion. Groups of tick larvae were immersed in defined concentrations of LGTV and eight to ten individual ticks from each group were examined for viral RNA by RT-PCR. As the concentration of virus increased from 105 to 107 pfu/ml, the percentage of ticks infected also increased with 100% of the ticks infected following immersion at 107 pfu/ml (Table 2). However, LGTV RNA was not detected in ticks following immersion in the lower concentrations of virus (102, 103 and 104 pfu/ml). To further quantify the infection efficiency following immersion in 107 pfu/ml, we examined an additional 65 larvae at 15 dpim. Infection was confirmed in 96% ±2 (mean ± SEM, n = 8 experiments) of the tick larvae (data not shown). These results demonstrated that the immersion protocol is an extremely efficient method of infecting I. scapularis larvae with LGTV.
Table 2.
Infection efficiency of I. scapularis larvae with LGTV following immersion
| Virus concentration
(pfu/ml) |
Percent infected
(# infected/total)a |
|---|---|
| 0 | 0 (0/10) |
| 102 | 0 (0/9) |
| 103 | 0 (0/10) |
| 104 | 0 (0/10) |
| 105 | 30 (3/10) |
| 106 | 75 (6/8) |
| 107 | 100 (8/8) |
Infection was determined by the presence of LGTV RNA in the tick at 15 dpim.
Quantification of replication in LGTV-infected I. scapularis larvae
To examine virus replication, we analyzed synchronously infected tick larva for the amplification of LGTV RNA using real-time PCR. Since the LGTV negative sense RNA strand, or complementary RNA is not a component of the virion, its presence is an obligatory marker for virus replication. Therefore, levels of RNA were determined using primers and probes specific for both the positive and the negative sense strands of LGTV RNA. Total RNA was isolated from groups of 10 larvae at 0, 3, 5, 10, and 15 dpim. Interestingly, some negative sense RNA was detected at 0 dpim. However, the virus stock, which was derived from the supernatant of infected cells, was found to contain the complementary RNA strand of LGTV (data not shown). This probably reflected negative sense strand RNA trapped in cellular debris from the lytic infection.
The changes in the positive and negative strands of LGTV RNA during infection of tick larvae were depicted in Figure 2. Both positive and negative sense LGTV RNA increased over the time course of infection. Importantly, the fact that the negative sense LGTV RNA increases is a strong indicator that LGTV replication is occurring in I. scapularis larvae after synchronous infection by immersion. This particular experiment was repeated with three separate sets of larvae and, although some variation in the relative fold-difference was noted, a consistent pattern of replication was observed. Specifically, two boosts occurred in virus replication following the 3 dpim and 10 dpim timepoints. This bi-phasic replication cycle in an arthropod vector may reflect an initial round of virus replication in the midgut with a subsequent round of replication in other secondary target organs (Foster and Jones, 1979).
Figure 2. LGTV replication in larvae following infection.
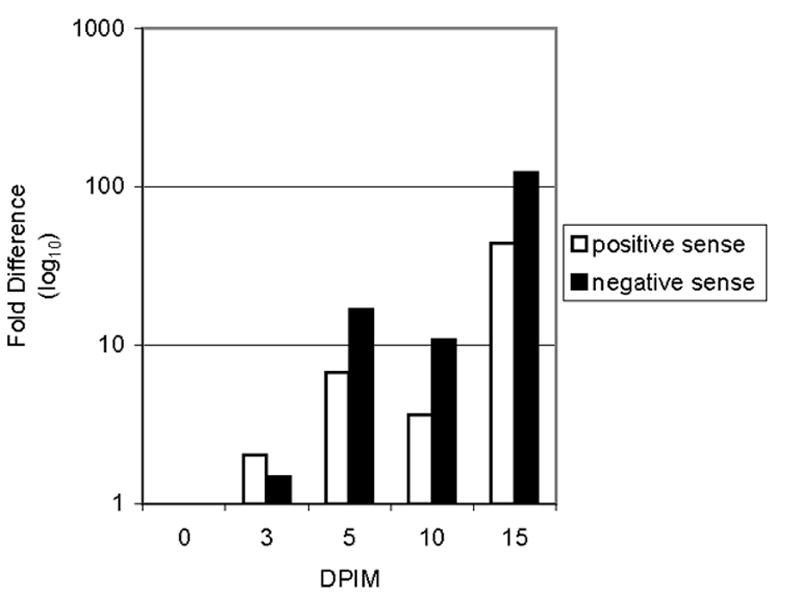
Total RNA was isolated from groups of 10 ticks immersed in LGTV at 0, 3, 5, 10 and 15 dpim. Real-time PCR was used to quantitate the changes in the positive and negative sense strands of LGTV RNA. Data at each timepoint was normalized to I. scapularis 16s rRNA. The data obtained at 0 dpim was used as the reference point for determining the fold difference.
Horizontal transmission of LGTV to mice following synchronous infection of I. scapularis tick larvae
For the immersion protocol to faithfully recapitulate the natural transmission cycle of LGTV, infected larvae must be competent to transmit the virus to a vertebrate host. Therefore, infected or mock-infected ticks were allowed to feed on 3 week old C57Bl/6 mice to engorgement. All mice exposed to the infected ticks had clear evidence of infection (Table 3). Some mice in every group developed characteristics of neurological disease, and viral infection was confirmed by the detection of positive strand LGTV RNA in the brains of these mice. However, a number of mice from each group survived without apparent neurological disease. To verify that these mice had a subclinical infection, we sought evidence of LGTV infection by looking for LGTV RNA in the brains of the surviving mice and for the presence of LGTV-specific antibody in the sera. All mice that survived infection following infestation of infected ticks seroconverted with antibody titers of at least 800. Additionally, LGTV RNA was detected in the brains of the surviving mice. Together, these results conclusively demonstrated that I. scapularis larvae infected by our immersion protocol are efficient at transmitting LGTV to 100% of our C57Bl/6 mouse model when the larvae feed to engorgement.
Table 3.
Horizontal transmission of LGTV from I. scapularis larvae to C57Bl/6 mice
| Moribund mice | Survivors | ||||
|---|---|---|---|---|---|
| Treatmenta | Placementb (dpim) |
% Mortality
(# dead / total mice)c |
Viral RNA
detected in brains d |
% Seroconversion
(# seroconverted / # survivors) |
Viral RNA
detected in brainsd |
| Mock | 17 | 0% (0/8) | - | 0% (0/8) | - |
| Infected | 3 | 37.5% (3/8) | + | 100% (5/5) | + |
| Infected | 5 | 12.5% (1/8) | + | 100% (7/7) | + |
| Infected | 10 | 75% (6/8) | + | 100% (2/2) | + |
| Infected | 17 | 50 % (4/8) | + | 100% (4/4) | + |
Ticks were immersed in media alone (Mock) or in media containing LGTV (Infected)
Ticks were placed on mice at 3, 5, 10 and 17 dpim.
Mice were considered terminal at the first signs of neurological disease.
Total RNA was isolated from the brains of mice and detection of viral RNA is represented by (+) presence, or (-) absence.
Transstadial transmission of LGTV following infection of larvae by immersion
Transstadial transmission is crucial for the maintenance of the tick-borne flaviviruses in nature. Thus, we wanted to determine if ticks infected by the immersion technique can maintain the virus infection from one stage to the next. Engorged larvae that had dropped off infested mice were collected and held for approximately one month until they molted into nymphs. Total RNA was isolated from individual nymphs and the viral RNA was amplified by RT-PCR. After molting, 87% ± 7.5 (mean ± SEM, 70 ticks in 7 experiments) of the nymphs were positive for LGTV RNA. Since 96% ± 2 (mean ± SEM) of the larvae were infected by the immersion technique, this equates to a transstadial transmission rate of approximately 92% (data not shown). These results demonstrated that ticks infected with LGTV by the immersion method can efficiently maintain the virus through molting from the larval to the nymphal stage.
To further evaluate transstadial transmission of LGTV, nymphs were examined for the presence of viral antigen by immunofluorescence following molting from larvae. LGTV antigen was readily detected in the hypodermis of the infected nymphs (Fig. 3a). Notably, viral antigen was not identified in this area of the tick prior to feeding. In addition to the hypodermis, LGTV was also detected in the salivary gland and the midgut of infected nymphs (Fig. 3). Conversely, no viral antigen was detected in mock infected nymphs. This widespread distribution of the virus in the above mentioned tissues subsequent to molting has been previously reported for ticks obtaining an infection via a bloodmeal (Nosek et al., 1986). These results not only confirm transstadial transmission of the virus, but also suggest that this method mimics the natural spread of the virus during molting.
Figure 3. Localization of LGTV antigen following transstadial transmission to nymphs.

Following infection by immersion and obtaining a bloodmeal the mock or LGTV-infected, engorged larvae were housed until molting to nymphs. Nymphs were then sectioned. Left Panel: Sections were stained for the presence of LGTV antigen (green) by immunofluorescence. DAPI (blue) was used to counterstain the nuclei. Right Panel: To show tick anatomy, H&E staining was performed on the same section used for immunofluorescent staining. LGTV antigen was observed in the (A) hypodermis (arrow), midgut (arrowhead) and (B) salivary gland (arrowhead) of the nymphs.
Discussion
Unlike many arthropod hosts, the lifespan of ticks from the egg through to the adult can last several years. Since tick-borne flaviviruses can persist in ticks throughout all stages of life, there are ample opportunities for the genotype and possibly the phenotype of the virus to be altered in the invertebrate host (Nuttall et al., 1991). As a result, understanding the interactions between the tick-borne flaviviruses and their tick vector is vital to developing in-depth concepts of viral pathogenesis and to devising strategies for control and prevention of the serious diseases that these viruses cause.
The technical limitations of the current methods used for infecting ticks restrict crucial studies of the virus-tick interactions. In order to study virus infection in ticks, we developed a novel method of synchronously infecting a large number of ticks with a defined virus stock by immersion. We demonstrated that the immersion technique leads to a high infection rate of I. scapularis larvae with LGTV and that viral replication ensues after the infection. Additionally, ticks infected by this method efficiently transmitted LGTV horizontally to a mouse model and transstadially to the nymph. For these reasons, the immersion technique will be an advantageous method for examining both the viral and tick determinants of replication, pathogenesis, and transmission.
The immersion technique utilizes the tick’s natural ability of maintaining its water balance by active water uptake. This mechanism of water uptake in unfed ixodid ticks transpires through the oral cavity (Rudolph and Knulle, 1974). Once imbibed through the orifice, the water is swallowed and proceeds to the gut (Kahl et al., 1990; Machin, 1979; Needham and Teel, 1986). This suggests that when ticks are immersed in media containing the virus, the virus would be imbibed through the oral cavity, swallowed and would then travel to the midgut, representing a route of infection similar to that of a natural infection obtained from a bloodmeal. Since this mechanism of water uptake has been demonstrated for the different life stages of the ixodid tick, this method should work well with not only larvae but also with nymphs and adult ticks (Kahl et al., 1990). We are currently examining the infection of I. scapularis nymphs with LGTV by the immersion technique.
Infecting ticks by feeding on a viremic vertebrate host imposes limitations which restrict studies involving the interactions between the virus and their tick vector. Exposing ticks to a viral infection by this method requires that the virus replicate in the host and attain a viremic titer that is sufficient to transmit the virus to a naive tick (Chernesky, 1969). Consequently, viruses that are impaired for replication in a vertebrate host cannot be readily studied in the tick system. Additionally, the amplification of the viruses in the vertebrate host allows for selective pressures to be imposed on the virus and can lead to selection of a minor virus genotype or to viral mutations (Dzhivanian et al., 1988; Kaluzova et al., 1994; Nuttall et al., 1991). Thus, the virus infecting the tick may differ from the initial inoculum in undefined ways after passage in the vertebrate host. Finally, when ticks feed on a vertebrate host, specific and non-specific host components (e.g. antibody and complement) are imbibed during the bloodmeal. These factors may have effects on the virus-vector interaction that are independent of the virus and difficult to control. Thus, by eliminating the need for an infected animal, the immersion technique offers several distinct advantages which will enable infection of ticks with an inoculum of a defined sequence, and will facilitate studies using attenuated strains.
Analysis performed by Leonova (1997) identified a correlation between the severity of the disease and the tick species prevalent at that time. This study suggests that the different tick species could select for tick-borne flavivirus strains of different virulence. The mechanisms involved in selection of viruses by the ticks are not known. However, virus selection maybe due to disparities in viral replication caused by differences in replication efficiency or by altered virus distribution within the tick. These factors could influence the viral load being transmitted to the vertebrate host, thereby affecting the pathogenesis. By combining the immersion method of infecting ticks with techniques such as real-time PCR and in situ hybridization, replication efficiencies and dissemination of the different virus strains can be examined among the various tick species. Variations in viral pathogenicity also could be associated with transmission. Using the system described in this paper, transmission efficiencies of the virus from the tick vector to the mouse model could be easily examined. Sequencing and annotation of an ixodid tick genome is currently underway (Hill and Wikel, 2005). As more information about the genome becomes available, the immersion technique may turn out to be a valuable system for examining interactions between viral and tick gene products that are important to both virus replication and transmission.
The highest infection efficiency was obtained when ticks were immersed in a virus stock containing 107 pfu/ml. One reason for the high titer requirement could be that ticks imbibe a very small quantity of media during the immersion protocol. Alternatively, the necessity for a high titer virus stock could reflect the vector efficiency of this particular tick species. Although a wide range of ixodid tick species can be infected with different members of the tick-borne flaviviruses (Chernesky and McLean, 1969; Costero and Grayson, 1996; Kozuch and Nosek, 1985; Nosek and Kozuch, 1985; Varma and Smith, 1972), I. scapularis is not the natural vector for LGTV and therefore may not be an efficient vector for this virus. Precedence for this concept of different efficiencies may be found from studies with another tick-borne flavivirus. Comparison between the Dermacentor andersoni and the I. pacificus ticks demonstrated that infection of I. pacificus with Powassan virus required a 100 fold increase in the viremic titer of the vertebrate host (Chernesky, 1969). The immersion technique now makes it possible to readily perform controlled comparative studies to examine the relative competence of individual tick species to infection with a specific virus.
Finally, we envision that the immersion technique may facilitate studies of other tick-borne viruses, such as Crimean-Congo hemorrhagic fever (CCHF) or Colorado tick fever. Furthermore, the high containment level at BSL-3 or BSL-4 required for some of these viruses like TBEV, CCHF, OMSK, and KFDV make experimental studies difficult. The current methods of infecting ticks by feeding on a viremic animal or by microinjection add another level of intricacy to the studies examining the interactions between the tick and the BSL-3 or BSL-4 viruses. Since the immersion technique does not require any special equipment and eliminates the need for both viremic animals and needles, this method will prove convenient for use in high containment working conditions.
Materials and Methods
Cells, viruses, and animals
A Vero cell-derived virus stock of Langat virus (LGTV) strain TP21 was provided by Dr. Alexander Pletnev (NIAID, NIH) (Pletnev and Men, 1998). The virus was further propagated in Vero cells (European Collection of Cell Culture) or in mouse neuroblastoma cells (MNB109) using a multiplicity of infection (MOI) of .005 (Campbell and Pletnev, 2000; Pletnev and Men, 1998). Virus titers were determined by plaque assay as previously described (Best et al., 2005). ISE6 cells, a cell line derived from I. scapularis embryonated eggs (a gift from Dr. Kurtti, University of Minnesota) were cultured as previously described (Munderloh et al., 1994). This tick cell line was infected with LGTV and used to create a standard curve for real-time PCR.
Adult C57Bl/6 mice were supplied by Jackson Laboratory and bred at Rocky Mountain Laboratory (RML). Twenty to 21 day old C57Bl/6 mice were used for experiments in accordance with the RML Institutional Animal Care and Use Committee.
Synchronous infection of ticks
I. scapularis larvae were maintained at a relative humidity of 98% at room temperature in a 16hr:8hr light:dark cycle, and were used within 6 months of emergence. Approximately 60 larvae were collected in a sterile 1.5 ml screw cap centrifuge tube and pretreated by exposure to a reduced relative humidity (P. Policastro, personal communication). Larvae were infected by immersion (Policastro and Schwan, 2003), essentially as described. Specifically, the ticks were suspended in 0.5 ml of complete Dulbecco’s modified eagle’s medium (DMEM) containing 1×107 pfu/ml (unless indicated differently) of LGTV and incubated at 34°C for 45 min. The tubes were vortexed every 10 min to redistribute the ticks in the media. After incubation, the tubes were chilled on ice for 2 min and centrifuged at 200 ×g for 30 s. The ticks were washed two times with cold phosphate-buffered saline (PBS) by centrifugation. Larvae were wicked free of excess moisture using tapered strips of Whatman paper and were maintained at a relative humidity of 98% until used as described below.
Immunofluorescence of tick midgut
Larvae and nymphs were fixed in 100% acetone at room temperature for 24 h and then stored in 70% ethanol. The cuticle was separated using #5 Dumont forceps under a dissecting stereomicroscope. The nymphs were dehydrated in a series of ethanol and then cleared three times in 100% xylene. The nymphs were infiltrated and embedded with paraffin, and then sectioned at 5 μm.
To prepare midguts for immunofluorescence, the midguts of the larvae were removed following separation of the cuticle and suspended in the Cryochrome, a colored mounting medium. In order to identify the placement of the midgut, the specimens were snap frozen and adhered to a prepared block of Cryomatrix, a white mounting medium. Frozen sections, cut at 6 μm, were mounted on slides and fixed with 100% acetone.
To prepare the slides for staining, sections of the whole tick were deparaffinized and rinsed with PBS. The frozen sections of the larval midgut were rinsed in distilled water to remove the mounting media. The sections were treated with a 3% hydrogen peroxide solution for 10 min to block endogenous peroxidase, rinsed with PBS, and then blocked in 5% normal goat serum in PBS for 20 min. The slides were rinsed with PBS and incubated with a polyclonal mouse antibody preparation that is reactive against the structural (E) and nonstructural (NS1, NS5) proteins of LGTV (hyperimmune mouse ascites fluid, clone Russian Spring Summer Encephalitis (RSSE) VR79; ATCC) at a 1:1000 dilution in PBS for 1 h at 37°C (Best et al., 2005). Following a PBS wash, the secondary antibody (goat anti-mouse IgG conjugated with Alexa Fluor 488; Invitrogen) was applied at a 1:1000 dilution in PBS and incubated for 30 min at 37°C. The slides were rinsed in PBS and coverslips were mounted using ProLong Gold antifade with 4’6-diamidino-2-phenylindole (DAPI; Invitrogen). To obtain images of the anatomical structures, the coverslips were removed following immunofluorescence and stained with hematoxylin and eosin. Images were captured using an Olympus BX51 microscope with an Olympus DP70 camera and Microsuite software.
Isolation of RNA
Total RNA from I. scapularis larvae or ISE6 cells was extracted using the RNeasy mini kit (Qiagen) (Schwaiger and Cassinotti, 2003). Ticks were frozen in liquid nitrogen and then triturated with an RNAse free plastic mortar and pestle (Fisher). The powder was resuspended in 250 μl of Buffer RLT. For the ISE6 cells, the infected cell pellet was resuspended in 350 μl of Buffer RLT. The ticks or cell lysates were homogenized by passing through a QIAshredder spin column (Qiagen). To prepare a positive control for RT-PCR, viral RNA was isolated from 5.6×106 pfu of an MNB cell derived virus stock using the QIAmp Viral RNA mini kit (Qiagen) following the manufacturer’s instructions.
In order to examine the presence of LGTV RNA in mouse brains, C57Bl/6 mice were deeply anesthetized with isoflurane and exsanguinated by transcardial perfusion with 20 ml of PBS. The brain was removed and bisected along the sagittal suture. Half of the brain was stored in RNA Later (Invitrogen), an RNA stabilization reagent, until RNA extraction was performed. The brain was homogenized in 4 ml of Buffer RLT (Qiagen) using the Omni TH homogenizer with the soft tissue disposable tips (Omni International). Total RNA was isolated using the RNeasy midi kit (Qiagen) as per the manufacturer’s instructions.
Amplification of RNA by RT-PCR
Viral RNA was detected using SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen). The RNA was reversed transcribed at 55°C for 30 min. PCR conditions used were 40 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 45 s (LGTV RNA) or at 43°C for 45 s (I. scapularis 16s ribosomal RNA (rRNA)), and extension at 68°C for 1 min. The primers used in the reactions are listed in Table 1.
Table 1.
Primers and probes used for RT-PCR and real-time RT-PCR
| Name | Sequence | Genome position |
|---|---|---|
| LGT4B5’ | 5’-AGACCAAGGGGAGACTGTGCGATGG-3’ | 6964-6988a |
| Lan4-3-4 | 5’-ACCACCTTGGCATGATAGGC-3’ | 9399-9380a |
|
| ||
| 16srRNA ISEF | 5’-CCTAATCCAACATCGAGGTC-3’ | 61-80b |
| 16s rRNA-ISER | 5’-ATGAGTGCTAAGAGAATGA-3’ | 372-354b |
|
| ||
| LangatprM-EFor | 5’-ATGGATTGTTGCCCAGGATTC-3’ | 909-929a |
| LangatprM-ERev | 5’-TTTCCAGGTGGGTGCATCTC-3’ | 992-973a |
| LangatprM-ETMp | 5’ 6FAM-CTCTCGCATTGGCACCGGCCTACG-TAMRA-3’ | 945-968a |
|
| ||
| LGTVnegstrandF | 5’-GTCTCCGGTTGCAGGACTGT-3’ | 6825-6806a |
| LGTVnegstrandR | 5’-CTCGGTCAGTAGGATGGTGTTG-3’ | 6685-6706a |
| LGTVnegstrand1 | 5’-6FAM-CCACAGGAAGATCAGTGAG-TAMRA-3’ | 6745-6727a |
|
| ||
| 16sF | 5’-ACCAAAAAAGAATCCTAATCCAACA-3’ | 48-72b |
| 16sR | 5’-GTTCCGTTTTTAGCGATTAAATGAA-3’ | 168-144b |
| Tick16s | 5’-6FAM-CGAGGTCGCAAACTATTTTATCTATATGAACTATCCAAAA-TAMRA-3’ | 74-113b |
Evaluation of virus replication by real-time PCR
RNA was DNAse treated for 1 h at 37°C and purified using the RNA cleanup protocol in the RNeasy mini kit (Qiagen). Ten fold serial dilutions from 1:10 to 1:10000 were performed on the RNA. Triplicate reactions were completed in a 10 μl volume using the One-Step reverse transcription-PCR master mix (Applied Biosystems), 500 nM of both the forward and reverse primer (Table 1), 250 nM of the probe, and 1.4 μl of RNA from the serial dilutions. The primers and probes used for detecting the positive sense strand RNA of LGTV (LangatprM-EFor, LangatprM-ERev, and LangatprM-ETMp), the negative sense strand RNA of LGTV (LGTVnegstrandF, LGTVnegstrandR, and LGTVnegstrand1) and the I. scapularis 16s rRNA (16sF, 16sR, and Tick16s) were designed using the Primer Express software version 2.0 (Applied Biosystems). RNA was analyzed with the ABI PRISM 7900 sequence detection system using the SDS2.2.2 software (Applied Biosystems).
The relative standard curve method was used to quantify the amount of viral RNA (Applied Biosystems User Bulletin #2). For development of the standard curves for LGTV and I. scapularis 16s rRNA, ISE6 cells were seeded at 4×106 cells in a 25 cm plate (Corning) and were infected with LGTV at an MOI of 1. Total RNA was isolated after 4 days of infection as described above and ten fold serial dilutions of the RNA were performed. To determine the fold difference, the amount of viral RNA was normalized to the I. scapularis 16s rRNA at each corresponding time point and the data at 0 dpim was used as the reference for obtaining fold differences.
Transmission studies
To examine virus transmission, the neck and shoulders of 20-21 day old C57Bl/6 mice were shaved, and approximately 100 mock-infected or LGTV-infected larvae were applied at different times post immersion with a small paintbrush. Each mouse was then confined in a wire tube wrapped with muslin cloth and the ticks were allowed to attach. After the ticks attached, the mice were housed in a wire-bottomed cage that was placed over a pan of water and the ticks were allowed to feed to engorgement (approximately 3-6 days). Using a paintbrush, the ticks were collected from the pan of water following engorgement and detachment from the mouse.
To evaluate horizontal transmission of LGTV to mice following infestation with infected ticks, the mice were observed daily for signs of disease which include weight loss, ruffled fur, hunchback posture, loss of balance, and hind-limb paralysis (Holbrook et al., 2005; Seamer and Randles, 1967). Animals demonstrating loss of balance or paralysis were considered terminal and were exsanguinated by transcardial perfusion with PBS and the brains were harvested for detection of viral RNA as described above. Mice surviving 28 days post attachment of ticks were considered to have survived the infection. Serum was collected from these mice by retro-orbital bleed and the mice were then exsanguinated and processed as described above.
To examine transstadial transmission across the tick stages, the engorged larvae were housed at 98% relative humidity until molting to the nymph stage occurred approximately 1 month after engorgement. Total RNA was isolated from the nymphs for the detection of viral RNA as noted above for tick larvae.
Enzyme-linked immunosorbent assay (ELISA)
Horizontal transmission was further evaluated by examining the sera of mice surviving 28 days post placement of ticks for the presence of LGTV-specific antibodies. The viral antigen for ELISA was prepared as described by Saeed et al. (2001). A 75 cm2 flask of LGTV infected Vero cells was harvested when 70% cytopathic effect was achieved, and the cells were resuspended in 10 ml of a 0.1M borate-saline solution (pH 9.0) containing 1% Triton x-100. The cell lysate was sonicated in a cup probe at room temperature three times for 15 s each and then centrifuged at 1854 ×g for 20 min at 4°C to pellet cell debris. To confirm that this procedure inactivated the virus, the viral antigen was blind-passaged three times through Vero cells and then plaqued on Vero cells as noted above. No residual virus was detected.
ELISAs were performed by coating a 96 well microtiter ELISA plates overnight with 50 ng of LGTV antigen. The plates were washed with PBS containing 0.5% Tween-20 (PBS-T) and then blocked with a 5% dehydrated milk solution in PBS-T (Blotto) for 2 h at 37°C. Two-fold serial dilutions of the test sera were made in PBS-T from 1:50 to 1:1600 and 100 μl were added to the wells in triplicate. The plates were incubated for 2 h at 37°C and then washed 6 times with PBS-T. One hundred microliters of the secondary antibody (1:1000 dilution in PBS-T of HRP-conjugated rabbit anti-mouse; DAKO) was added to the wells and incubated at 37°C for 30 min. The plate was washed as before and the signal was developed with 100 μl of a 0.1M NaOAc (pH=5), 0.12mg/ml 3,3’,5,5’ tetramethylbenzidine (TMB) and 0.015% hydrogen peroxide solution for 1 h at room temperature. The reaction was stopped using 50 μl of 2M sulfuric acid and the absorbance at 450 nm was measured using the MRX microplate reader (Dynatech Laboratories). Titers were determined to be the reciprocal of the highest dilution having an OD450 absorbance that was at least 3 fold above the background absorbance reading.
Acknowledgments
The authors thank Drs. Tom Schwan and Paul Policastro for providing I. scapularis larvae and technical support, Drs. Sue Priola, Rachel LaCasse, Paul Policastro, and Tom Schwan for critical review of the manuscript, and Gary Hettrick and Anita Mora for graphical help. This research was supported by the intramural research program of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J Virol. 2005;79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MS, Pletnev AG. Infectious cDNA clones of Langat tick-borne flavivirus that differ from their parent in peripheral neurovirulence. Virology. 2000;269:225–237. doi: 10.1006/viro.2000.0220. [DOI] [PubMed] [Google Scholar]
- Chernesky MA. Powassan virus transmission by ixodid ticks infected after feeding on viremic rabbits injected intravenously. Can J Microbiol. 1969;15:521–526. doi: 10.1139/m69-090. [DOI] [PubMed] [Google Scholar]
- Chernesky MA, McLean DM. Localization of Powassan virus in Dermacentor andersoni ticks by immunofluorescence. Can J Microbiol. 1969;15:1399–1408. doi: 10.1139/m69-252. [DOI] [PubMed] [Google Scholar]
- Costero A, Grayson MA. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae) Am J Trop Med Hyg. 1996;55:536–546. doi: 10.4269/ajtmh.1996.55.536. [DOI] [PubMed] [Google Scholar]
- Dzhivanian TI, Korolev MB, Karganova GG, Lisak VM, Kashtanova GM. Changes in the host-dependent characteristics of the tick-borne encephalitis virus during its adaptation to ticks and its readaptation to white mice. Vopr Virusol. 1988;33:589–595. [PubMed] [Google Scholar]
- Foster NM, Jones RH. Multiplication rate of bluetongue virus in the vector Culicoides variipennis (Diptera: Ceratopogonidae) infected orally. J Med Entomol. 1979;15:302–303. doi: 10.1093/jmedent/15.3.302. [DOI] [PubMed] [Google Scholar]
- Gaunt MW, Sall AA, de L X, Falconar AK, Dzhivanian TI, Gould EA. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J Gen Virol. 2001;82:1867–1876. doi: 10.1099/0022-1317-82-8-1867. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antiviral Res. 2003;57:129–146. doi: 10.1016/s0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Holbrook MR, Aronson JF, Campbell GA, Jones S, Feldmann H, Barrett AD. An animal model for the tickborne flavivirus--Omsk hemorrhagic fever virus. J Infect Dis. 2005;191:100–108. doi: 10.1086/426397. [DOI] [PubMed] [Google Scholar]
- Jones LD, Davies CR, Steel GM, Nuttall PA. Vector capacity of Rhipicephalus appendiculatus and Amblyomma variegatum for Thogoto and Dhori viruses. Med Vet Entomol. 1989;3:195–202. doi: 10.1111/j.1365-2915.1989.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Kahl O, Hoff R, Knulle W. Gross morphological changes in the salivary glands of Ixodes ricinus (Acari, Ixodidae) between bloodmeals in relation to active uptake of atmospheric water vapour. Exp Appl Acarol. 1990;9:239–258. doi: 10.1007/BF01193431. [DOI] [PubMed] [Google Scholar]
- Kaluzova M, Eleckova E, Zuffova E, Pastorek J, Kaluz S, Kozuch O, Labuda M. Reverted virulence of attenuated tick-borne encephalitis virus mutant is not accompanied with the changes in deduced viral envelope protein amino acid sequence. Acta Virol. 1994;38:133–140. [PubMed] [Google Scholar]
- Kozuch O, Nosek J. Replication of tick-borne encephalitis (TBE) virus in Ixodes ricinus ticks. Folia Parasitol (Praha) 1985;32:373–375. [PubMed] [Google Scholar]
- Lawrie CH, Uzcategui NY, Armesto M, Bell-Sakyi L, Gould EA. Susceptibility of mosquito and tick cell lines to infection with various flaviviruses. Med Vet Entomol. 2004;18:268–274. doi: 10.1111/j.0269-283X.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- Leonova GN. Tick-borne Encephalitis in Primorskii Region: Virological, Ecological, and Epidemiological Aspects. Dal’nuaka Press; Vladivostok: 1997. [Google Scholar]
- Machin J. Atmospheric water absorption in arthropods. Adv Insect Physiol. 1979;14:1–48. [Google Scholar]
- Munderloh UG, Liu Y, Wang M, Chen C, Kurtti TJ. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–543. [PubMed] [Google Scholar]
- Needham GR, Teel PD. Water balance by ticks between bloodmeals. In: Sauer JR, Hair JA, editors. Morphology, Physiology, and Behavioral Biology of Ticks. Ellis Horwood Limited; Chichester: 1986. pp. 100–151. [Google Scholar]
- Nosek J, Chunikhin SP, Gresikova M, Korolev MB, Kozuch O, Stefutkina LF, Ivannikova TI. Peculiarities of tick-borne encephalitis virus reproduction in Haemaphysalis inermis ticks and their explants. Acta Virol. 1986;30:396–401. [PubMed] [Google Scholar]
- Nosek J, Kozuch O. Replication of tick-borne encephalitis (TBE) virus in ticks Dermacentor marginatus. Angew Parasitol. 1985;26:97–101. [PubMed] [Google Scholar]
- Nuttall PA, Jones LD, Davies CR. The Role of Arthropod Vectors in Arbovirus Evolution. Advances in Disease Vector Research. 1991:15–45. [Google Scholar]
- Nuttall PA, Labuda M. Dynamics of infection in tick vectors and at the tick-host interface. Adv Virus Res. 2003;60:233–272. doi: 10.1016/s0065-3527(03)60007-2. [DOI] [PubMed] [Google Scholar]
- Pletnev AG, Men R. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc Natl Acad Sci USA. 1998;95:1746–1751. doi: 10.1073/pnas.95.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Knulle W. Site and mechanism of water vapour uptake from the atmosphere in ixodid ticks. Nature. 1974;249:84–85. doi: 10.1038/249084a0. [DOI] [PubMed] [Google Scholar]
- Saeed MF, Nunes M, Vasconcelos PF, Travassos Da Rosa AP, Watts DM, Russell K, Shope RE, Tesh RB, Barrett AD. Diagnosis of Oropouche virus infection using a recombinant nucleocapsid protein-based enzyme immunoassay. J Clin Microbiol. 2001;39:2445–2452. doi: 10.1128/JCM.39.7.2445-2452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol. 2003;27:136–145. doi: 10.1016/s1386-6532(02)00168-3. [DOI] [PubMed] [Google Scholar]
- Seamer J, Randles WJ. The course of Langat virus infection in mice. Br J Exp Pathol. 1967;48:403–410. [PMC free article] [PubMed] [Google Scholar]
- Steele GM, Nuttall PA. Difference in vector competence of two species of sympatric ticks, Amblyomma variegatum and Rhipicephalus appendiculatus, for Dugbe virus (Nairovirus, Bunyaviridae) Virus Res. 1989;14:73–84. doi: 10.1016/0168-1702(89)90071-3. [DOI] [PubMed] [Google Scholar]
- Varma MG, Smith CE. Multiplication of Langat virus in the tick Ixodes ricinus. Acta Virol. 1972;16:159–167. [PubMed] [Google Scholar]
- Xu G, Fang QQ, Keirans JE, Durden LA. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol. 2003;89:452–457. doi: 10.1645/0022-3395(2003)089[0452:MPAITT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]



