Abstract
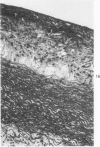
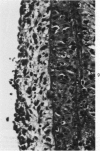
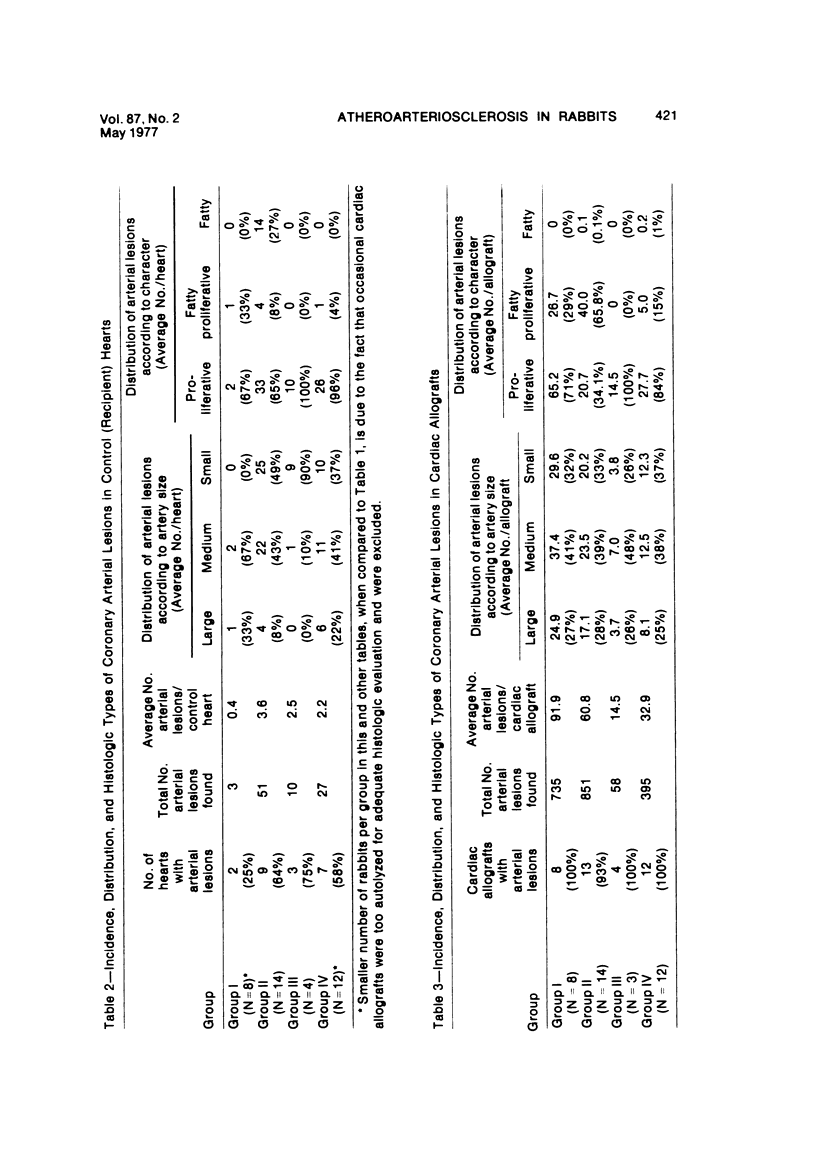
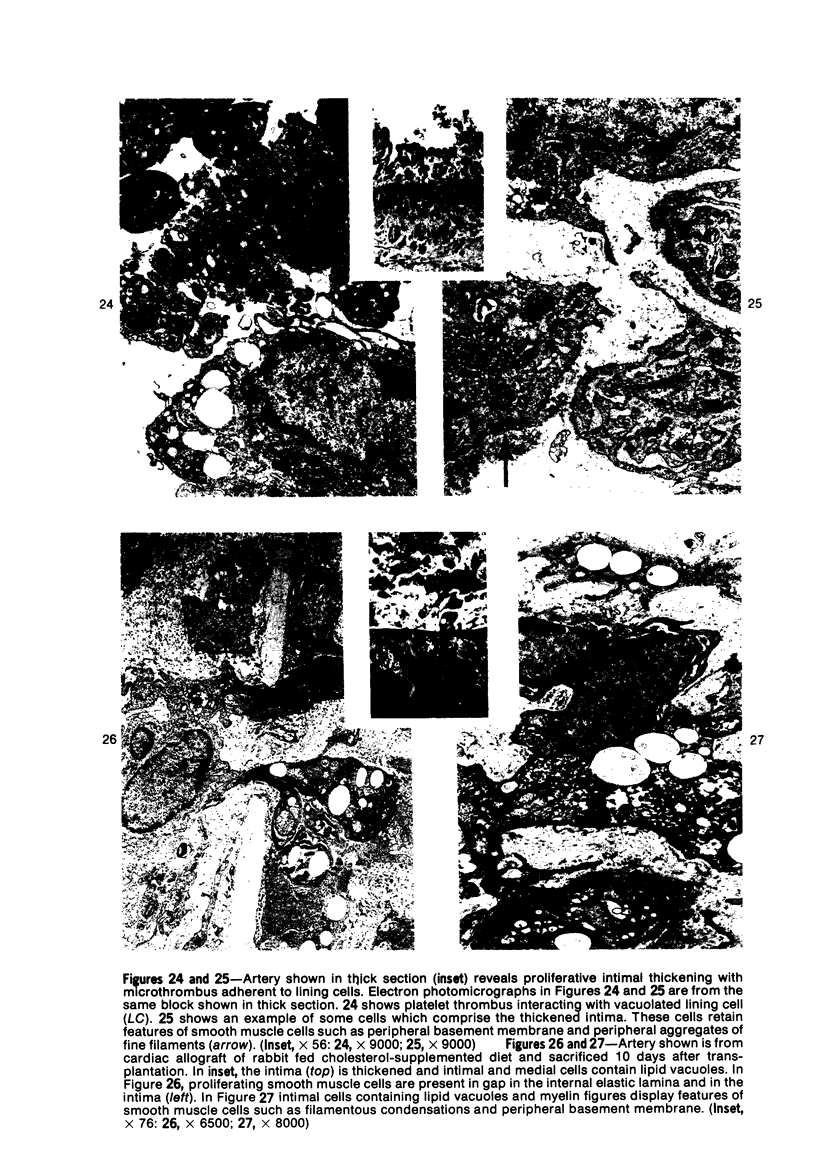
Heterotopic cardiac allografts were placed in the necks of 48 rabbits. In rabbits that were not immunosuppressed, allografts beat as long as 12 days, while in immunosuppressed rabbits allografts beat as long as 101 days. Coronary arterial lesions in donor hearts of rabbits fed a lipid-poor diet were found in arteries of all sizes and were mainly proliferative without fatty change. In cholesterol-fed rabbits, arterial lesions were similarly distributed, but the majority of lesions in longer surviving transplants were fatty-proliferative and some bore close resemblance to chronic human coronary atheroselerosis. In contrast to findings in cardiac homotransplants, only occasional predominantly fatty lesions were induced in small intramyocardial arteries of cholesterol-fed recipients. By electron microscopy, early arterial lesions in allografts were characterized by platelet aggregates in widened junctions between endothelial cells, sloughing of endothelium without intimal thickening but with adherence of platelets to the denuded arterial wall, and platelets deep within essentially normal media. Platelets were also seen adhering to the lining cells overlying the thickened intima of more advanced arterial lesions. Results indicate that immunologic arterial injury due to allograft rejection acting in synergy with hypercholesterolemia resulting from a dietary supplement of cholesterol can lead to rapidly developing atherosclerosis. Observations of early and evolving lesions indicate that endothelial injury and platelet interaction with the arterial wall are early and continuing events and may be of primary importance in the pathogenesis of experimental graft-induced atheroarteriosclerosis. In man, similar mechanisms may be involved in the pathogenesis of graft-induced athererosclerosis and in other instances of atherosclerosis. (Am J Pathol 87:415-442, 1977).
Full text
PDF

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- Alonso D. R., Scheidt S., Post M., Killip T. Pathophysiology of cardiogenic shock. Quantification of myocardial necrosis, clinical, pathologic and electrocardiographic correlations. Circulation. 1973 Sep;48(3):588–596. doi: 10.1161/01.cir.48.3.588. [DOI] [PubMed] [Google Scholar]
- Bieber C. P., Stinson E. B., Shumway N. E., Payne R., Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation. 1970 May;41(5):753–772. doi: 10.1161/01.cir.41.5.753. [DOI] [PubMed] [Google Scholar]
- Busch G. J., Martins A. C., Hollenberg N. K., Wilson R. E., Colman R. W. A primate model of hyperacute renal allograft rejection. Am J Pathol. 1975 Apr;79(1):31–56. [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G. STUDIES ON THE LOCALIZATION OF CIRCULATING ANTIGEN-ANTIBODY COMPLEXES AND OTHER MACROMOLECULES IN VESSELS. II. PATHOGENETIC AND PHARMACODYNAMIC STUDIES. J Exp Med. 1963 Oct 1;118:503–513. doi: 10.1084/jem.118.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Habal M., Hollenberg N. K., Birtch A. G., Busch G. J. Hyperacute renal allograft rejection in the primate. Therapeutic limitations of antiplatelet agents alone and combined with heparin. Am J Pathol. 1976 Jan;82(1):25–42. [PMC free article] [PubMed] [Google Scholar]
- Fisher-Dzoga K., Chen R., Wissler R. W. Effects of serum lipoproteins on the morphology, growth, and metabolism of arterial smooth muscle cells. Adv Exp Med Biol. 1974;43(0):299–311. doi: 10.1007/978-1-4684-3243-5_15. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Forbes R. D., Kuramochi T., Guttmann R. D., Klassen J., Knaack J. A controlled sequential morphologic study of hyperacute cardiac allograft rejection in the rat. Lab Invest. 1975 Sep;33(3):280–288. [PubMed] [Google Scholar]
- Friedman R. J., Moore S., Singal D. P. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975 Mar;32(3):404–415. [PubMed] [Google Scholar]
- Hardin N. J., Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. 3. The role of earlier acquired fibromuscular intimal thickening in the pathogenesis of later developing atherosclerosis. Am J Pathol. 1973 Nov;73(2):301–326. [PMC free article] [PubMed] [Google Scholar]
- Kniker W. T., Cochrane C. G. The localization of circulating immune complexes in experimental serum sickness. The role of vasoactive amines and hydrodynamic forces. J Exp Med. 1968 Jan 1;127(1):119–136. doi: 10.1084/jem.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek J. C., Bieber C., Lower R. R. Heart graft arteriosclerosis. Transplant Proc. 1971 Mar;3(1):512–514. [PubMed] [Google Scholar]
- Laden A. M. Experimental atherosclerosis in rat and rabbit cardiac allografts. Arch Pathol. 1972 Mar;93(3):240–245. [PubMed] [Google Scholar]
- Laden A. M. The effects of treatment on the arterial lesions of rat and rabbit cardiac allografts. Transplantation. 1972 Mar;13(3):281–290. doi: 10.1097/00007890-197203000-00014. [DOI] [PubMed] [Google Scholar]
- Lee K. T., Jarmolych J., Kim D. N., Grant C., Krasney J. A., Thomas W. A., Bruno A. M. Production of advanced coronary atherosclerosis, myocardial infarction and "sudden death" in swine. Exp Mol Pathol. 1971 Oct;15(2):170–190. doi: 10.1016/0014-4800(71)90097-9. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Lee K. T. Advanced coronary atherosclerosis in swine produced by combination of balloon-catheter injury and cholesterol feeding. Exp Mol Pathol. 1975 Dec;23(3):491–499. doi: 10.1016/0014-4800(75)90039-8. [DOI] [PubMed] [Google Scholar]
- MacSween R. N., Ono K., Thomason C. M. Immunological and histological studies of ectopic heart transplants in the rat. J Pathol. 1971 Sep;105(1):49–56. doi: 10.1002/path.1711050107. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E., Campbell W. G., Jr Experimental induction of athero-arteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. I. Effect of repeated injections of horse serum in rabbits fed a dietary cholesterol supplement. J Exp Med. 1966 Oct 1;124(4):635–652. doi: 10.1084/jem.124.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. II. Effect of repeatedly injected foreign protein in rabbits fed a lipid-rich, cholesterol-poor diet. Am J Pathol. 1973 Nov;73(2):265–300. [PMC free article] [PubMed] [Google Scholar]
- PORTER K. A., CALNE R. Y., ZUKOSKI C. F. VASCULAR AND OTHER CHANGES IN 200 CANINE RENAL HOMOTRANSPLANTS TREATED WITH IMMUNO-SUPPRESSIVE DRUGS. Lab Invest. 1964 Jul;13:810–824. [PubMed] [Google Scholar]
- Rider A. K., Copeland J. G., Hunt S. A., Mason J., Specter M. J., Winkle R. A., Bieber C. P., Billingham M. E., Dong E., Jr, Griepp R. B. The status of cardiac transplantation, 1975. Circulation. 1975 Oct;52(4):531–539. doi: 10.1161/01.cir.52.4.531. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. M., Rosensweig J., Chatterjee S., Moore S., De Champlain M. L. Platelets in hyperacute rejection of heterotopic cardiac allografts in presensitized dogs. An experimental study. Am J Pathol. 1973 Feb;70(2):155–174. [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B. Vascular intimal components: precursors of thrombosis. Prog Hemost Thromb. 1974;2(0):1–47. [PubMed] [Google Scholar]
- Thomson J. G. Production of severe atheroma in a transplanted human heart. Lancet. 1969 Nov 22;2(7630):1088–1092. doi: 10.1016/s0140-6736(69)90700-4. [DOI] [PubMed] [Google Scholar]