Abstract
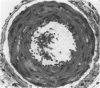
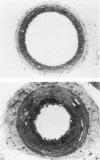
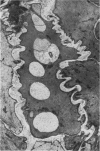
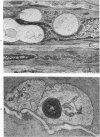
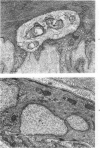
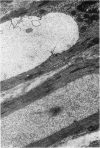
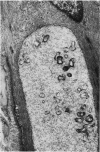
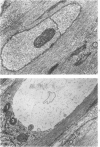
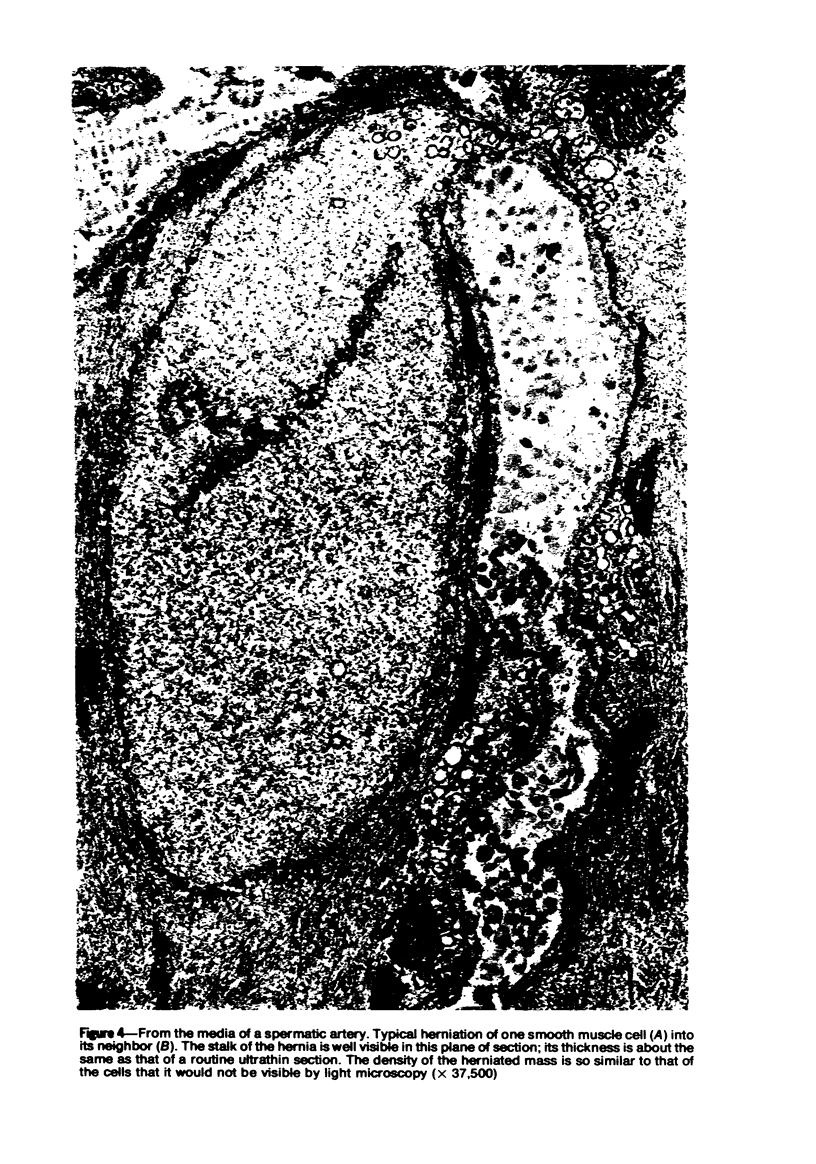
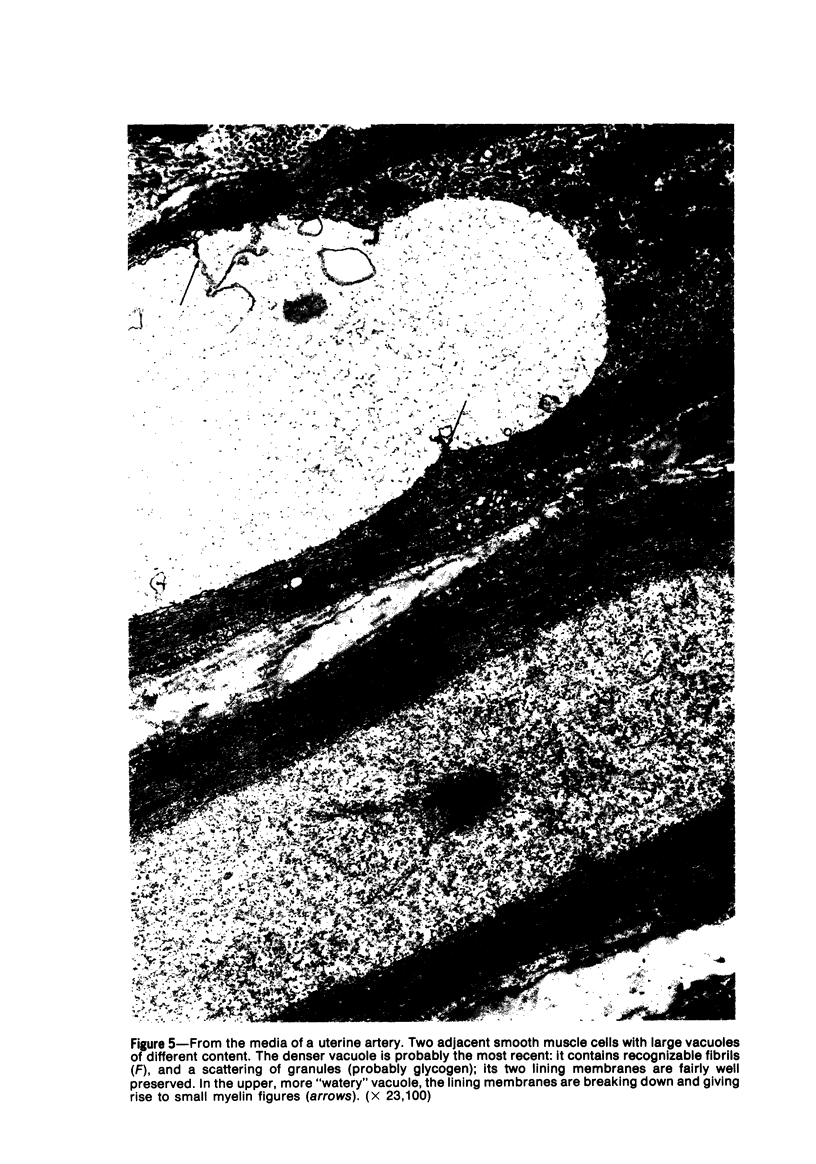
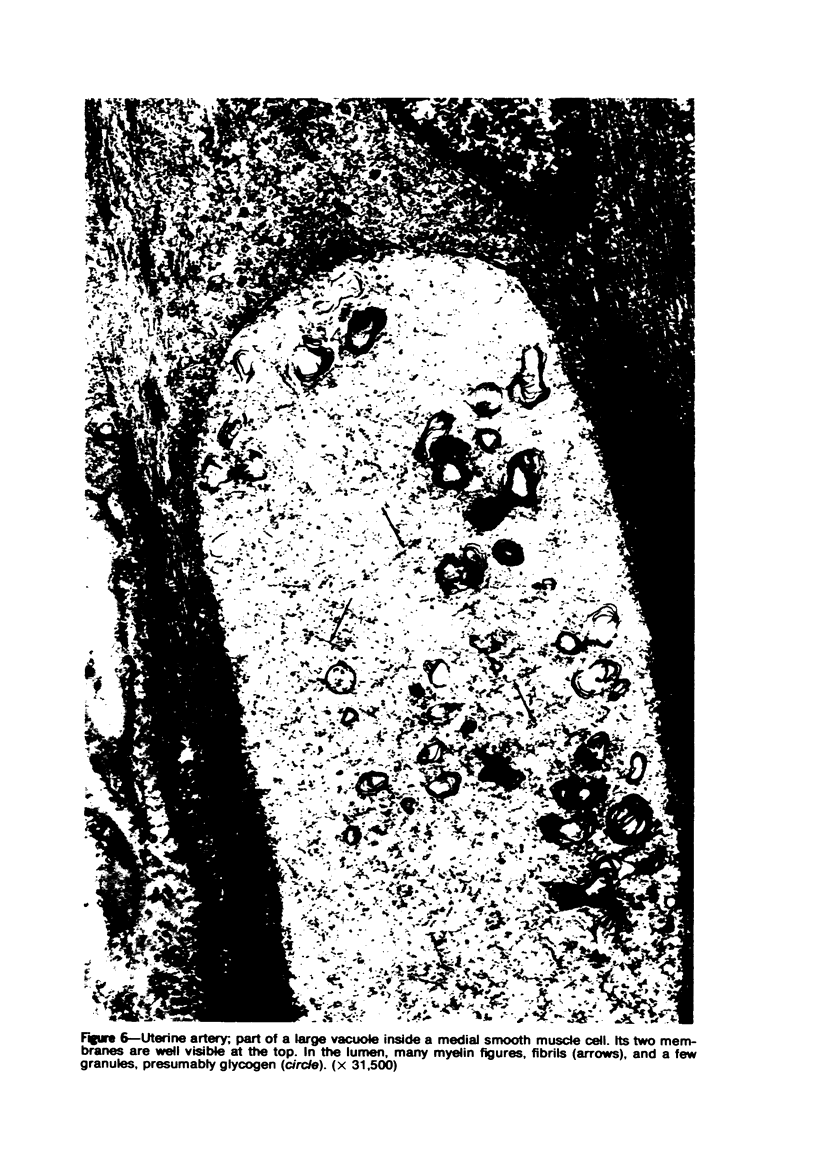
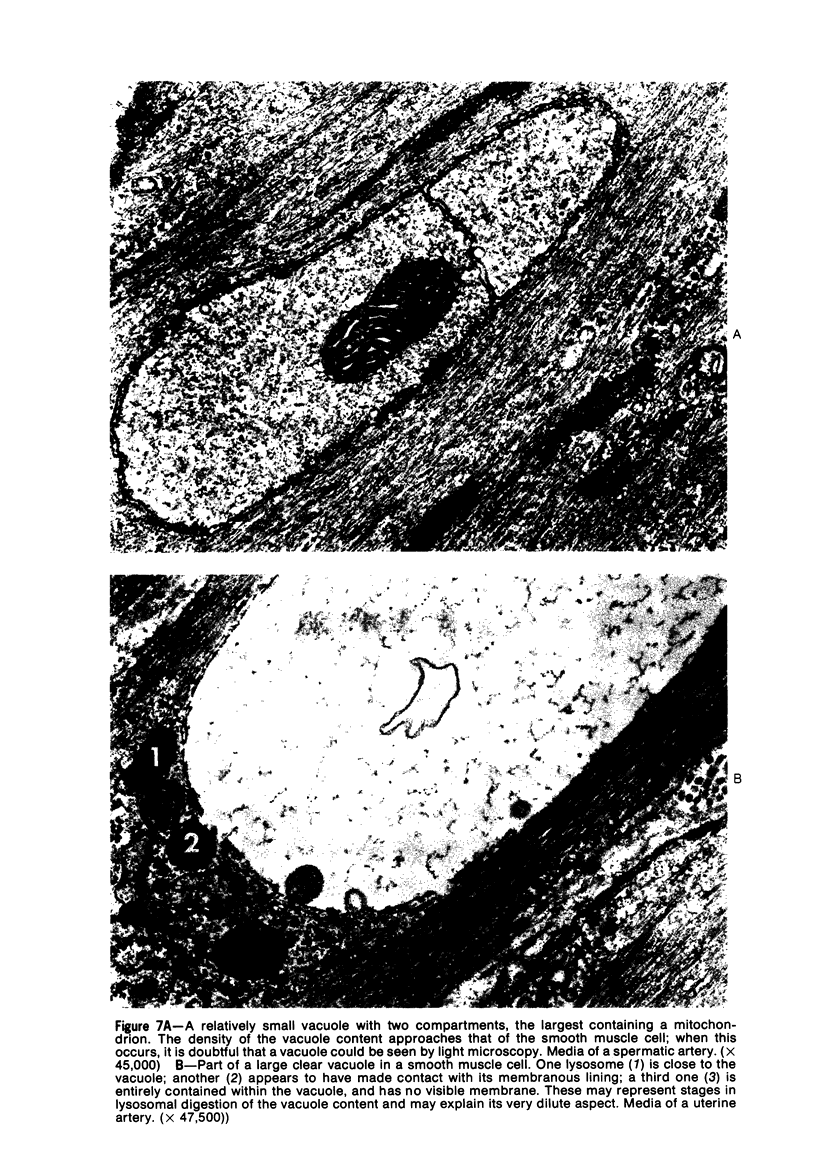
Vacuoles were observed by light microscopy in the smooth muscle cells of the media in normal rat arteries. By electron microscopy these vacuoles were limited by two membranes; they usually contained myelin figures, a few organelles (especially mitochondria and microfilaments), and an amorphous background material that varied greatly in density. Morphologic evidence indicates that these structures arise by herniation of one smooth muscle cell into another; it is presumed that herniation occurs during contraction at weak points corresponding to areas where adjacent cells come in close contact. Such cell-to-cell herniae were mostly seen in small arteries (arterioles) with a diameter of 0.4 to 0.2 mm; however, none was found in coronary arteries of this size. This discrepancy suggests that the pathogenesis of cell-to-cell herniae is correlated not only with the caliber of the artery but also with functional demands. (Am J Pathol 87:375-398).
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke P. H., Smith S. C. Smooth muscle cells: the source of foam cells in atherosclerotic white Carneau pigeons. Exp Mol Pathol. 1968 Apr;8(2):171–189. doi: 10.1016/0014-4800(68)90014-2. [DOI] [PubMed] [Google Scholar]
- De la Iglesia F. A., Lumb G. Ultrastructural and circulatory alterations of the myocardium in experimental coronary artery narrowing. Lab Invest. 1972 Jul;27(1):17–31. [PubMed] [Google Scholar]
- Fay F. S., Delise C. M. Contraction of isolated smooth-muscle cells--structural changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Cliff W. J. The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest. 1975 May;32(5):585–600. [PubMed] [Google Scholar]
- Henderson R. M. Types of cell contacts in arterial smooth muscle. Experientia. 1975 Jan 15;31(1):103–105. doi: 10.1007/BF01924703. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Gottlob R. A fine structure study of injury to the endothelial cells of the rabbit abdominal aorta by various stimuli. Angiology. 1967 Jul;18(7):440–451. doi: 10.1177/000331976701800704. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., McDonald L. W., Hayes T. L. An electron microscope study of the rabbit aortic intima after occlusion by brief exposure to a single ligature. Br J Exp Pathol. 1968 Feb;49(1):68–73. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Majno G. Cellular breakdown within the arterial wall. An ultrastructural study of the coronary artery in young and aging rats. Virchows Arch A Pathol Anat Histol. 1974;364(1):111–127. doi: 10.1007/BF01230861. [DOI] [PubMed] [Google Scholar]
- Kosek J. C., Chartrand C., Hurley E. J., Lower R. R. Arteries in canine cardiac homografts. Ultrastructure during acute rejection. Lab Invest. 1969 Oct;21(4):328–335. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B. P. Alterations in the cytologic detail of intestinal smooth muscle cells in various stages of contraction. J Cell Biol. 1965 Oct;27(1):199–213. doi: 10.1083/jcb.27.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T. Tridimensional views of the relationship of pericytes to endothelial cells of capillaries in the human choroid and retina. J Electron Microsc (Tokyo) 1975;24(1):13–18. [PubMed] [Google Scholar]
- Matthews M. A., Gardner D. L. The fine structure of the mesenteric arteries of the rat. Angiology. 1966 Dec;17(12):902–931. doi: 10.1177/000331976601701203. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Sandberg L. B., Ross R., Layman D. L. The smooth muscle cell. III. Elastin synthesis in arterial smooth muscle cell culture. J Cell Biol. 1976 Mar;68(3):411–419. doi: 10.1083/jcb.68.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. The occurrence of inclusions within membrane-limited structures that run longitudinally in the cells of mammalian heart muscle. J Ultrastruct Res. 1967 Jan;17(1):63–71. doi: 10.1016/s0022-5320(67)80020-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODIN J. A. Fine structure of vascular walls in mammals with special reference to smooth muscle component. Physiol Rev Suppl. 1962 Jul;5:48–87. [PubMed] [Google Scholar]
- Ratliff N. B., Kopelman R. I., Goldner R. D., Cruz P. T., Hackel D. B. Formation of myocardial zonal lesions. Am J Pathol. 1975 May;79(2):321–334. [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI Y., OSADA M., WATANABE A. [Cytological and electron microscopical observations on the vascular system of the various organs and mesentery of guinea pigs, with special reference to the endothelial cells]. Arch Histol Jpn. 1962 Jul;22:477–514. doi: 10.1679/aohc1950.22.477. [DOI] [PubMed] [Google Scholar]