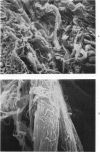
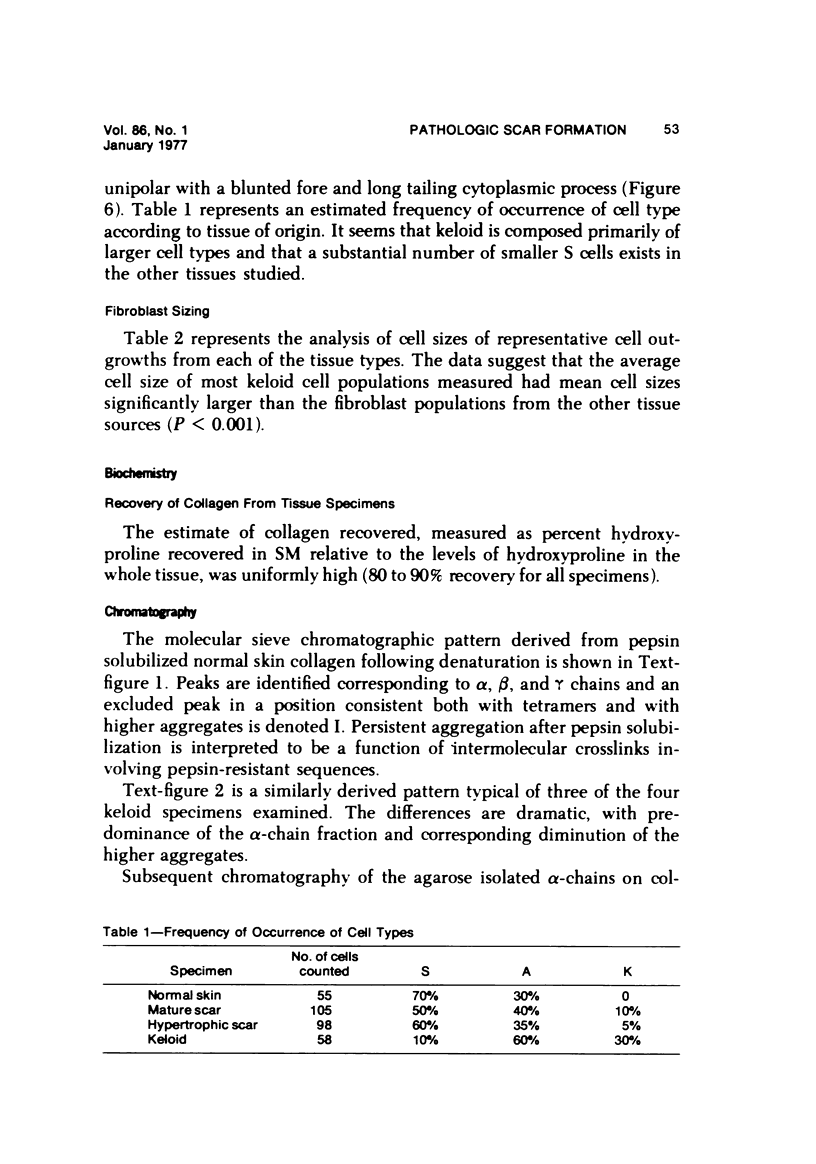
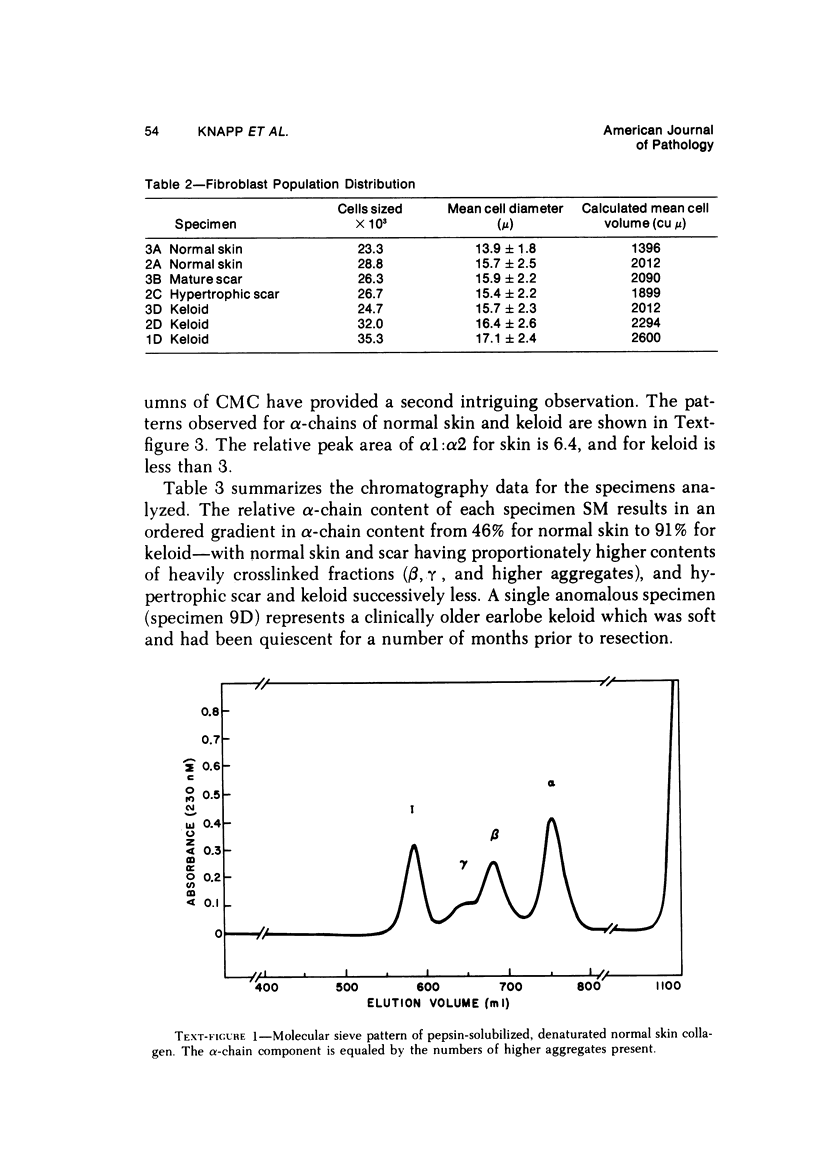
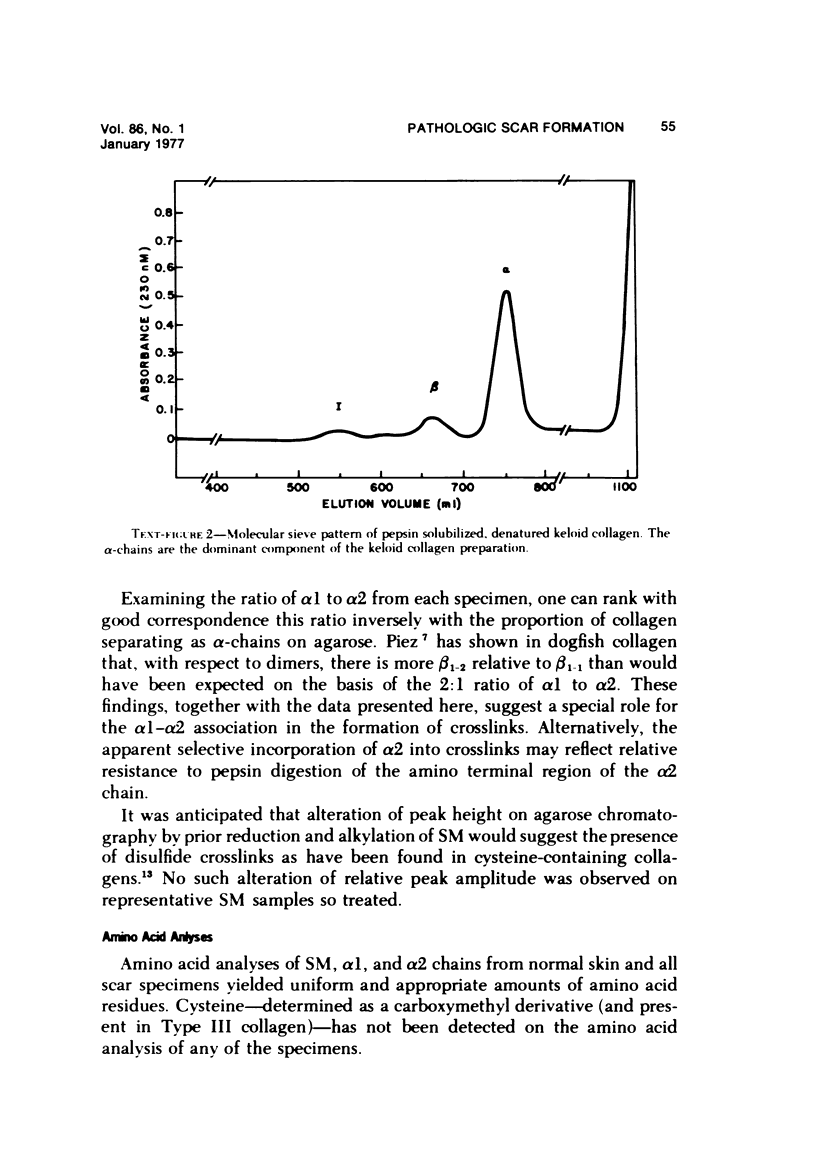
Abstract
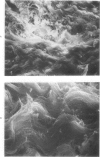
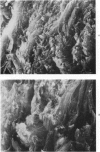
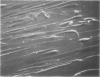
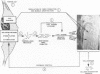
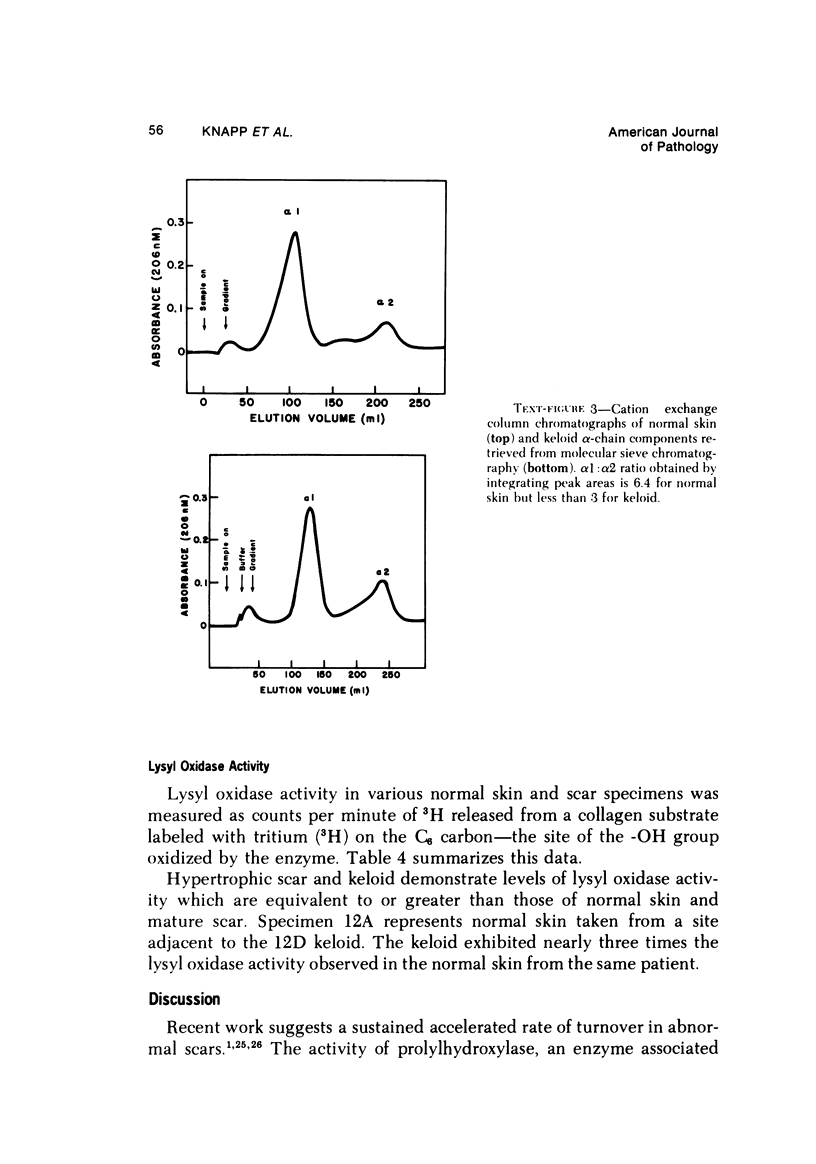
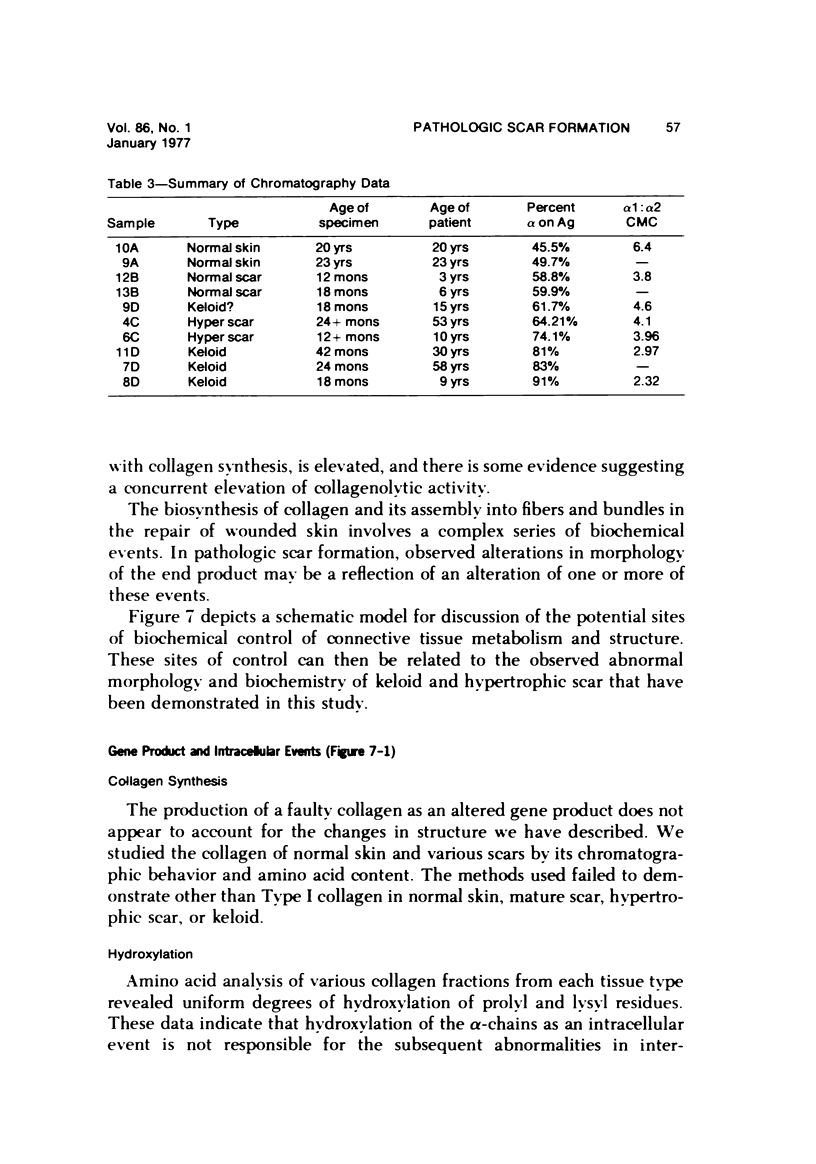
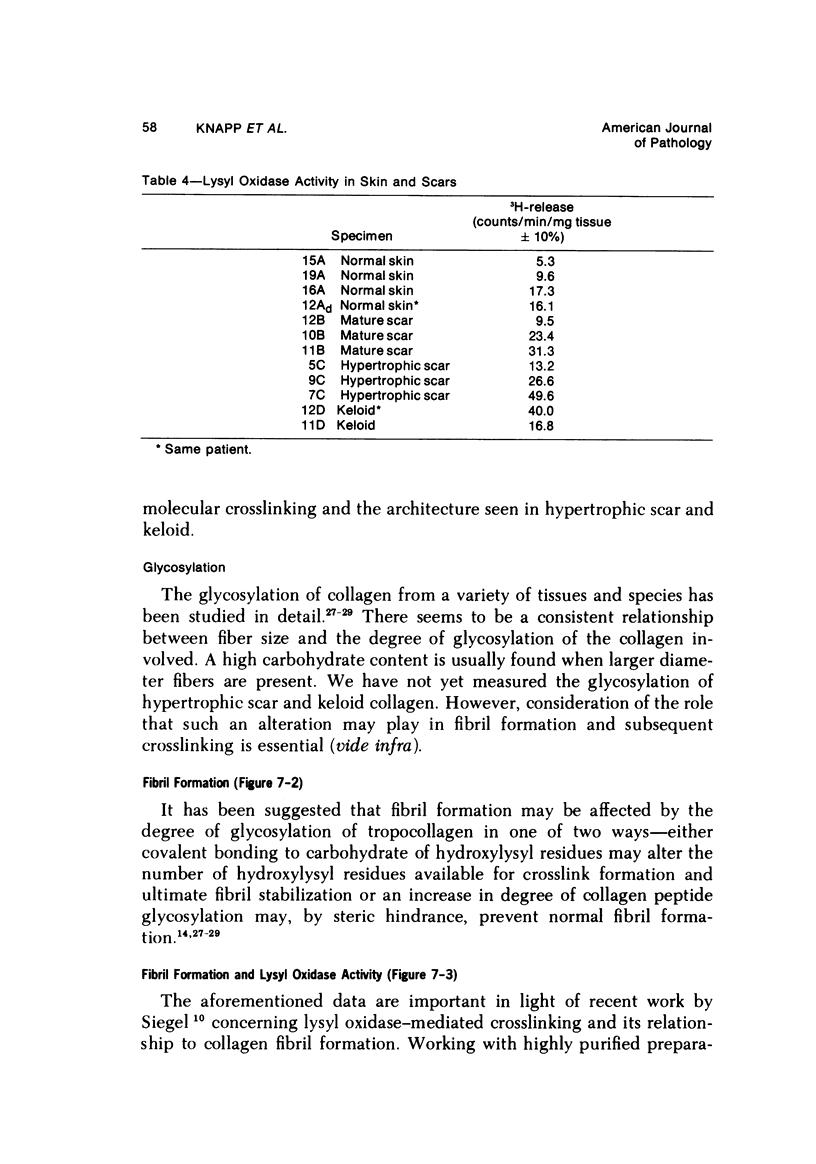
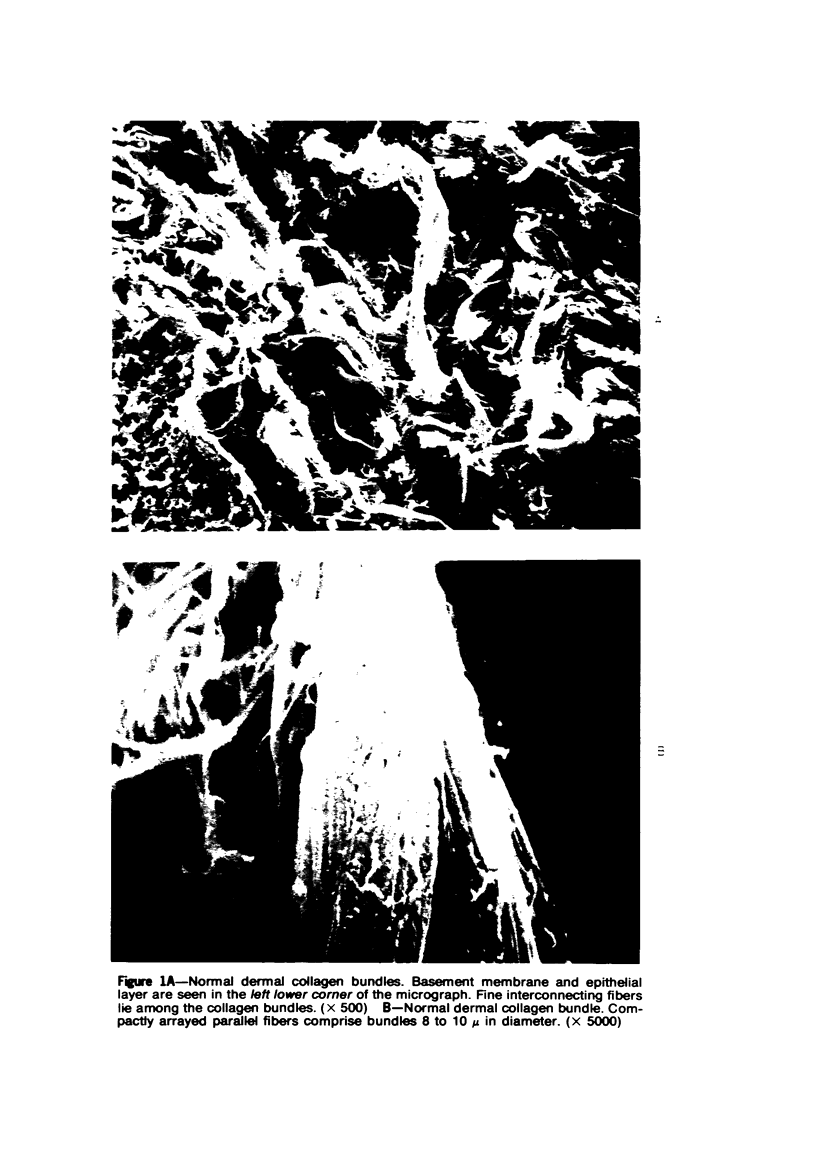
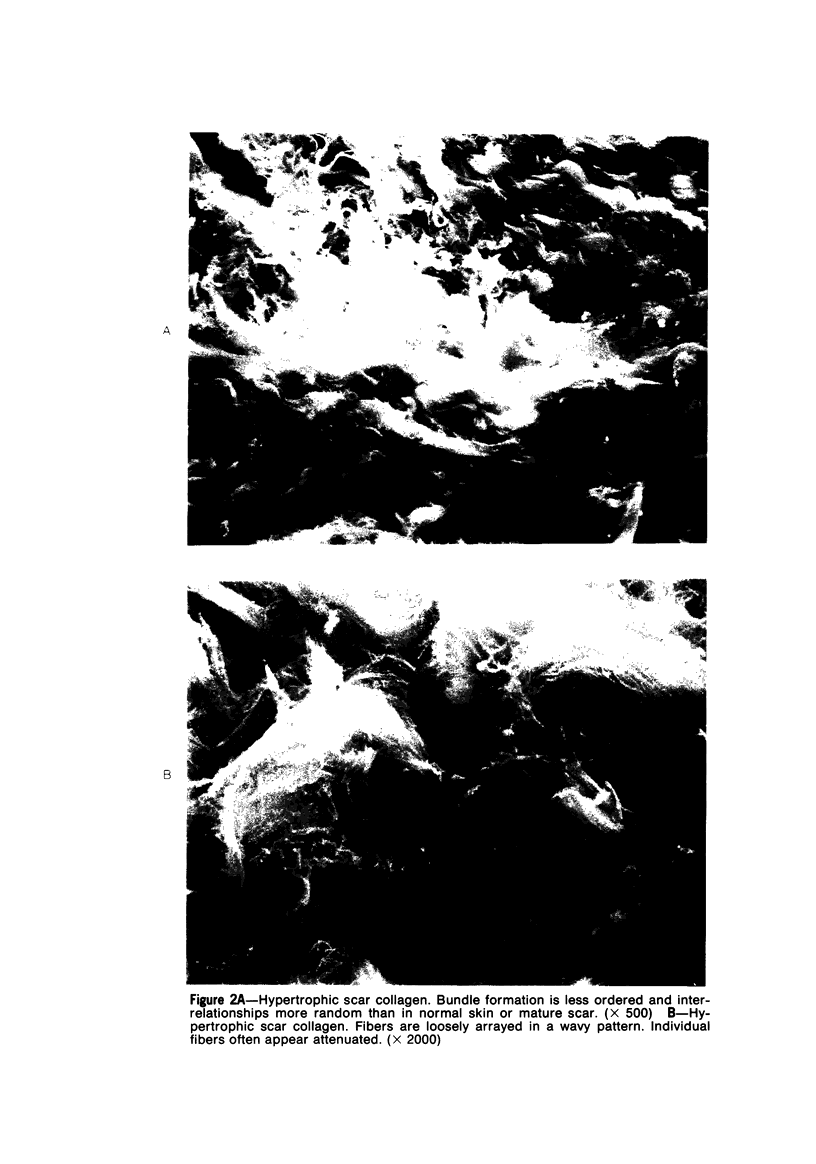
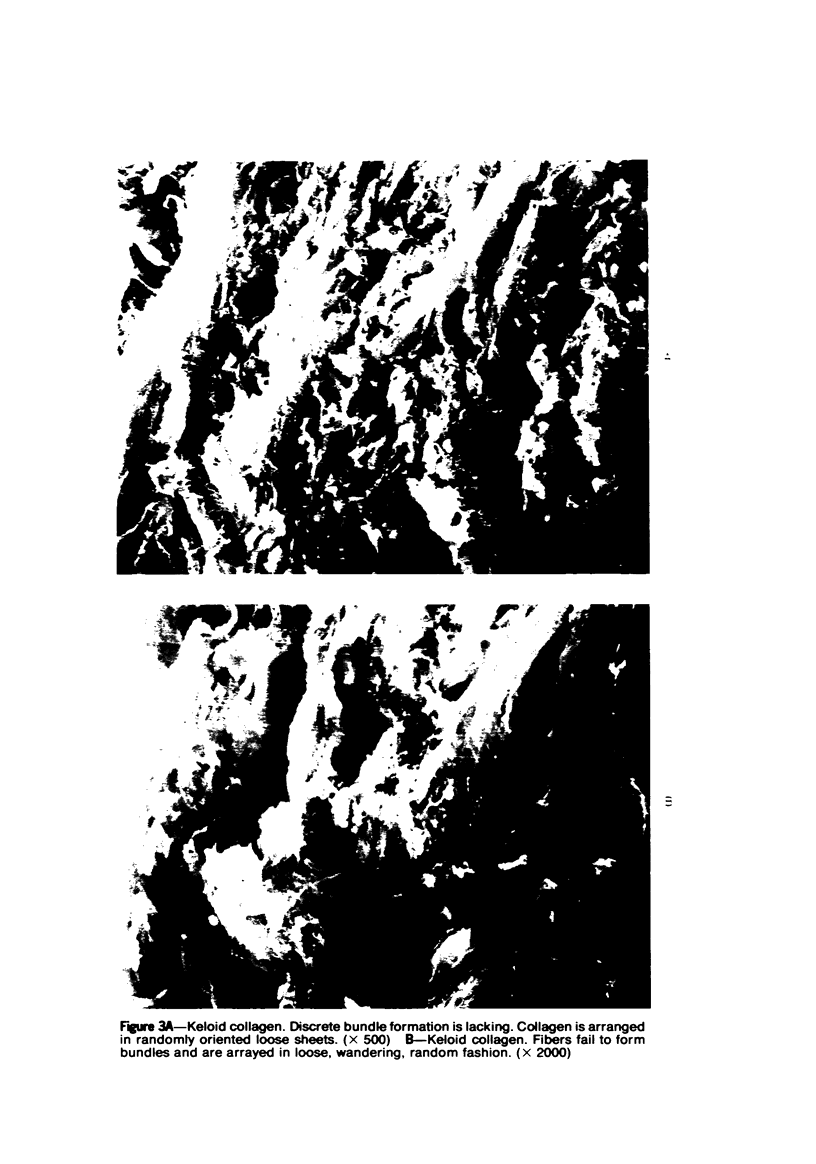
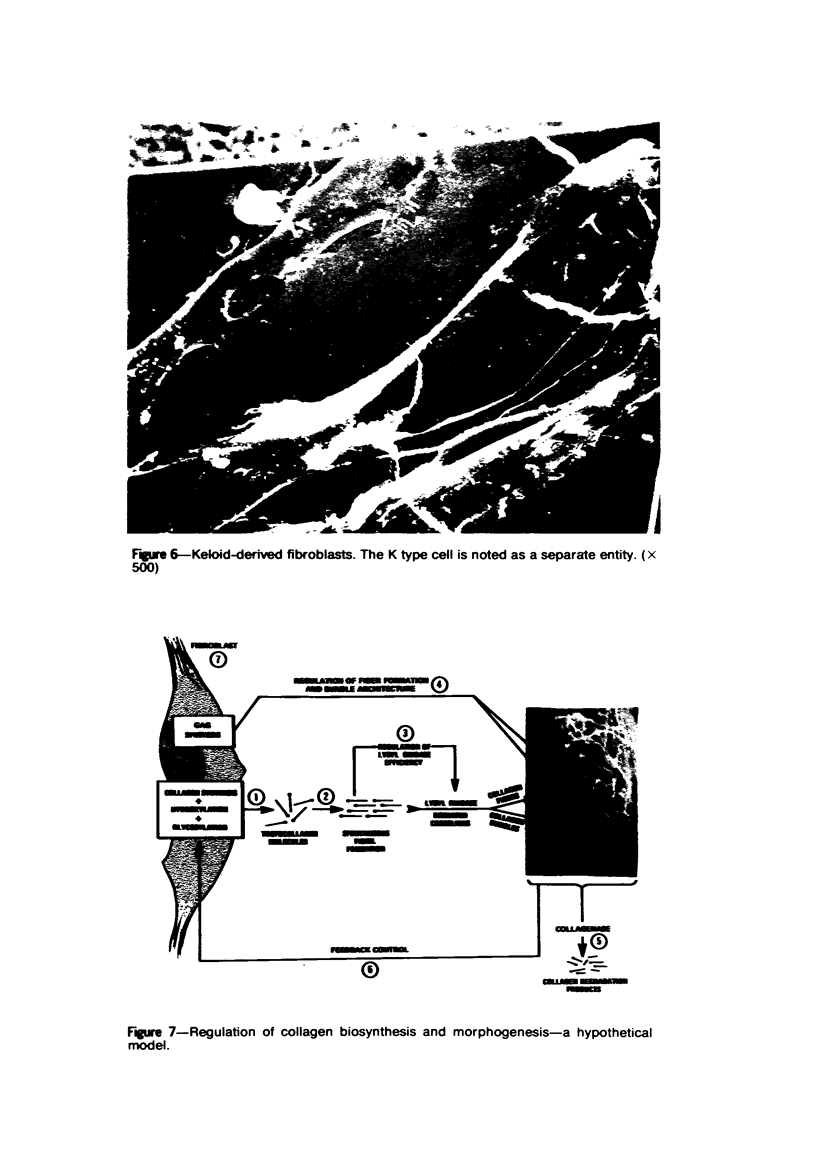
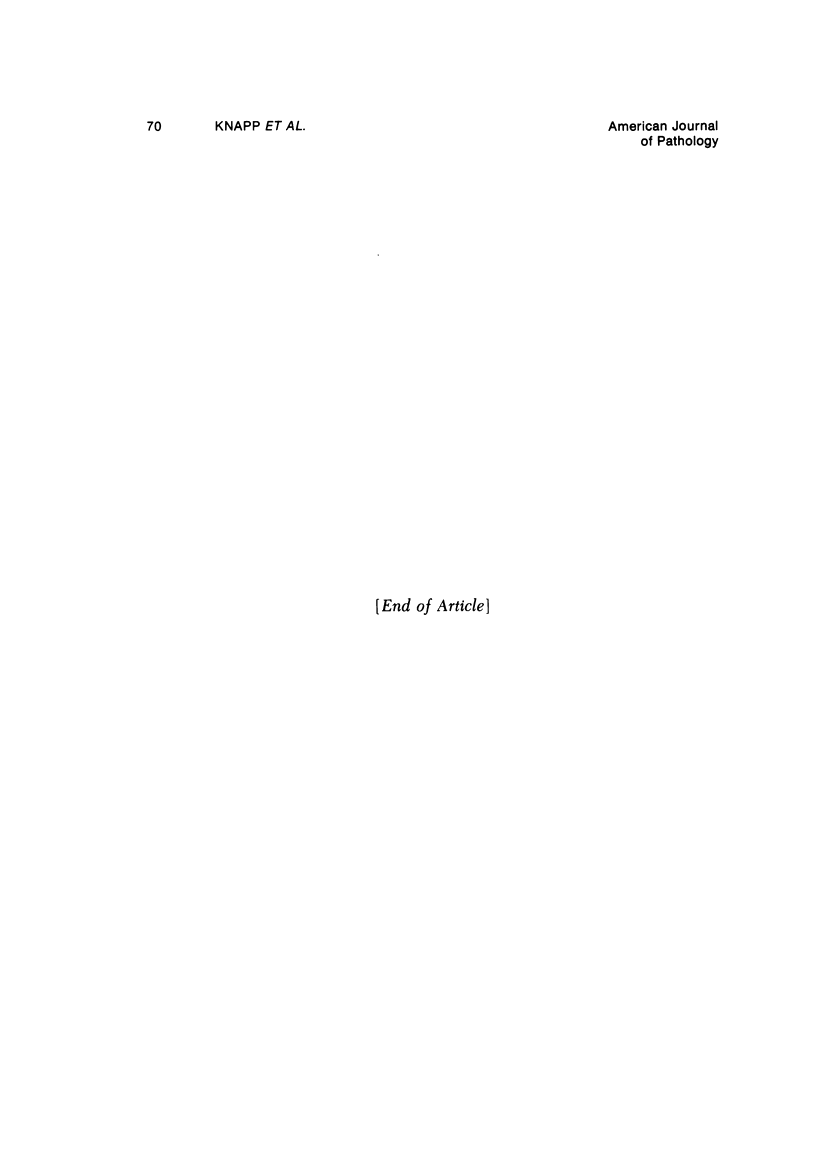
Morphologic and biochemical analyses were performed to compare normal skin and mature scars to hypertrophic scar and keloid. Correlation of morphologic findings with biochemical profiles of the skin and scar samples proved feasible and enlightening. Scanning electron microscopy (SEM) was used to characterize the architectural arrangement of collagen fibers in skin and scars. Cultured fibroblasts from each specimen were also examined with the SEM. A biochemical profile of each tissue specimen was constructed, characterizing the collagen component of the specimen by sequential molecular sieve and ion exchange chromatography to determine a) the degree of intermolecular crosslinking, b) amino acid analysis, and c) levels of lysyl oxidase activity. Results indicate that collagen fibers and fiber bundles display a decreasing level of organization as the clinical degree of scar abnormality increases, and this structural gradient correlates with the gradient of intermolecular crosslinking in the same tissue--normal skin and mature scar being highly crosslinked, hypertrophic and keloid successively less so. Surprisingly, the level of the crosslinking enzyme lysyl oxidase is normal or elevated in hypertrophic scar and keloid despite the relative lack of crosslinking. Amino acid content was uniform for all specimens. Scanning electron microscopy examination of cultured fibroblasts from the tissue specimens demonstrated three phenotypically distinctive fibroblasts whose numerical and volumetric proportions correlated with the tissue of origin.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., Beaven M. A., Horáková Z., Keiser H. R. Histamine and collagen synthesis in keloid and hypertrophic scar. Surg Forum. 1972;23(0):509–510. [PubMed] [Google Scholar]
- Cohen I. K., Keiser H. R., Sjoerdsma A. Collagen synthesis in human keloid and hypertrophic scar. Surg Forum. 1971;22:488–489. [PubMed] [Google Scholar]
- Daniels J. R., Chu G. H. Basement membrane collagen of renal glomerulus. J Biol Chem. 1975 May 10;250(9):3531–3537. [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. II. Functional groups involved in the interaction process. J Biol Chem. 1969 Apr 10;244(7):1787–1795. [PubMed] [Google Scholar]
- Donoff R. B., McLennan J. E., Grillo H. C. Preparation and properties of collagenases from epithelium and mesenchyme of healing mammalian wounds. Biochim Biophys Acta. 1971 Mar 10;227(3):639–653. doi: 10.1016/0005-2744(71)90014-3. [DOI] [PubMed] [Google Scholar]
- Drake M. P., Davison P. F., Bump S., Schmitt F. O. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry. 1966 Jan;5(1):301–312. doi: 10.1021/bi00865a039. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z. Human skin collagenase: localization and distribution in normal human skin. J Invest Dermatol. 1969 May;52(5):442–448. doi: 10.1038/jid.1969.76. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Finlay J. B., Hunter J. A., Steven F. S. Preparation of human skin for high-resolution scanning electron microscopy using phosphate buffered crude bacterial alpha-amylase. J Microsc. 1971 Feb;93(1):73–76. doi: 10.1111/j.1365-2818.1971.tb02267.x. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Freeman I. L., Schofield J. D., Jackson D. S. Variations in the carbohydrate content of human and bovine polymeric collagens from various tissues. Biochim Biophys Acta. 1969 May 6;177(3):682–685. doi: 10.1016/0304-4165(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 2. N Engl J Med. 1972 Feb 3;286(5):242–249. doi: 10.1056/NEJM197202032860505. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Spiro R. G. Fractionation of glycoprotein components of the reduced alkylated renal glomerular basement membrane. J Biol Chem. 1972 Jul 10;247(13):4239–4247. [PubMed] [Google Scholar]
- KEECH M. K. The formation of fibrils from collagen solutions. IV. Effect of mucopolysaccharides and nucleic acids: an electron microscope study. J Biophys Biochem Cytol. 1961 Jan;9:193–209. doi: 10.1083/jcb.9.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. H., Nagai Y., Piez K. A., Gross J. Studies on the structure of collagen utilizing a collagenolytic enzyme from tadpole. Biochemistry. 1966 Feb;5(2):509–515. doi: 10.1021/bi00866a016. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Ketchum L. D., Cohen I. K., Masters F. W. Hypertrophic scars and keloids. A collective review. Plast Reconstr Surg. 1974 Feb;53(2):140–154. doi: 10.1097/00006534-197402000-00004. [DOI] [PubMed] [Google Scholar]
- Kischer C. W., Shetlar M. R. Collagen and mucopolysaccharides in the hypertrophic scar. Connect Tissue Res. 1974;2(3):205–213. doi: 10.3109/03008207409152245. [DOI] [PubMed] [Google Scholar]
- Merrill J. T., Veizades N., Hulett H. R., Wolf P. L., Herzenberg L. A. An improved cell volume analyzer. Rev Sci Instrum. 1971 Aug;42(8):1157–1163. doi: 10.1063/1.1685332. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Morgan P. H., Jacobs H. G., Segrest J. P., Cunningham L. W. A comparative study of glycopeptides derived from selected vertebrate collagens. A possible role of the carbohydrate in fibril formation. J Biol Chem. 1970 Oct 10;245(19):5042–5048. [PubMed] [Google Scholar]
- Nimni M. E. Metabolic pathways and control mechanisms involved in the biosynthesis and turnover of collagen in normal and pathological connective tissues. J Oral Pathol. 1973;2(4):175–202. doi: 10.1111/j.1600-0714.1973.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C., Pinnell S. R., Martin G. R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970 Nov 10;9(23):4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Igarashi S., Gross J. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970 Dec 8;9(25):4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]
- Wood G. C. The precipitation of collagen fibers from solution. Int Rev Connect Tissue Res. 1964;2:1–31. doi: 10.1016/b978-1-4831-6751-0.50007-0. [DOI] [PubMed] [Google Scholar]