Abstract
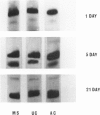
Acinar epithelial cells were purifed from suspensions of cells from the lactating mammary glands of rats. As described previously, this purififaction was accomplished by sedimentation in an isokinetic gradient of Ficoll (polysucrose) in tissue culture medium, and the purity of the separated cells was established by electron microscopy and by histochemical markers. Isoenzymes of glucose-6-phosphate dehydrogenase were investigated at various intervals during lactation in separated populations of stromal and acinar cells. Acinar cells contained three- to eightfold more glucose-6-phosphate dehydrogenase activity than did stromal cells. The proportions of the respective isoenzymes varied during the course of lactation, and the observed changes were parallel in purified acinar cells and in the lactating mammary glands from which the cell suspensions were obtained. The availability of purified acinar cells in the investigation of interactions between hormones and cells from the mammary gland permits a greater degree of specificity than has been possible in the study of mammary cell suspensions which contain myoepithelial cells, duct cells, acinar cells, endothelial cells, fibroblasts, fat cells, mast cells, plasma cells, and blood cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dietz A. A., Lubrano T. Separation and quantitation of lactic dehydrogenase isoenzymes by disc electrophoresis. Anal Biochem. 1967 Aug;20(2):246–257. doi: 10.1016/0003-2697(67)90030-9. [DOI] [PubMed] [Google Scholar]
- Fishman W. H. Perspectives on alkaline phosphatase isoenzymes. Am J Med. 1974 May;56(5):617–650. doi: 10.1016/0002-9343(74)90631-7. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L. Studies on mammary gland involution. I. On the ultrastructure of the lactating mammary gland. J Ultrastruct Res. 1968 Nov;25(3):193–213. doi: 10.1016/s0022-5320(68)80069-3. [DOI] [PubMed] [Google Scholar]
- Hilf R., Rector W., Abraham S. A glucose-6-phosphate dehydrogenase isoenzyme characteristic of preneoplastic and neoplastic mouse mammary tissue. J Natl Cancer Inst. 1973 May;50(5):1395–1398. doi: 10.1093/jnci/50.5.1395. [DOI] [PubMed] [Google Scholar]
- Jensen H., Schiodt T. Stromal response in breast carcinoma and fibroadenomatosis, estimate by the aid of the alkaline phosphatase activity. Acta Pathol Microbiol Scand A. 1971;79(4):321–329. doi: 10.1111/j.1699-0463.1971.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Kinsella J. E. The incorporation of [14C3]glycerol into lipids by dispersed bovine mammary cells. Biochim Biophys Acta. 1968 Dec 18;164(3):540–549. doi: 10.1016/0005-2760(68)90183-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murad T. M. A proposed histochemical and electron microscopic classification of human breast cancer according to cell of origin. Cancer. 1971 Feb;27(2):288–299. doi: 10.1002/1097-0142(197102)27:2<288::aid-cncr2820270207>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Murad T. M., Von Haam E. Studies of mammary carcinoma induced by 7,12-dimethylbenz(a)anthracene administration. Cancer Res. 1972 Jul;32(7):1404–1415. [PubMed] [Google Scholar]
- O'Keefe E., Cuatrecasas P. Insulin receptors in murine mammary cells: comparison in pregnant and nonpregnant animals. Biochim Biophys Acta. 1974 Mar 20;343(1):64–77. doi: 10.1016/0304-4165(74)90239-6. [DOI] [PubMed] [Google Scholar]
- Oka T., Perry J. W. Studies on the function of glucocorticoid in mouse mammary epithelial cell differentiation in vitro. Stimulation of glucose 6-phosphate dehydrogenase. J Biol Chem. 1974 Jun 10;249(11):3586–3591. [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Dynamics of insulin action on mammary epithelium. Nat New Biol. 1972 Oct 18;239(94):216–217. doi: 10.1038/newbio239216a0. [DOI] [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Insulin-sepharose and the dynamics of insulin action. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2066–2068. doi: 10.1073/pnas.68.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Is prolactin mitogenic for mammary epithelium? Proc Natl Acad Sci U S A. 1972 Jul;69(7):1693–1696. doi: 10.1073/pnas.69.7.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens I. S., Vonderhaar B. K., Topper Y. J. Concerning the necessary coupling of development to proliferation of mouse mammary epithelial cells. J Biol Chem. 1973 Jan 25;248(2):472–477. [PubMed] [Google Scholar]
- Ozols R. F., Hilf R. Effect of androgen on glucose-6-phosphate dehydrogenase isoenzymes in rat ventral prostate and seminal vesicles. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):73–77. doi: 10.3181/00379727-144-37530. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Dow S. R., Murad T. M., Jones J. Separation of acinar cells from lactating mammary gland using velocity sedimentation in an isokinetic gradient of Ficoll in tissue culture medium. Am J Pathol. 1974 Jul;76(1):95–106. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
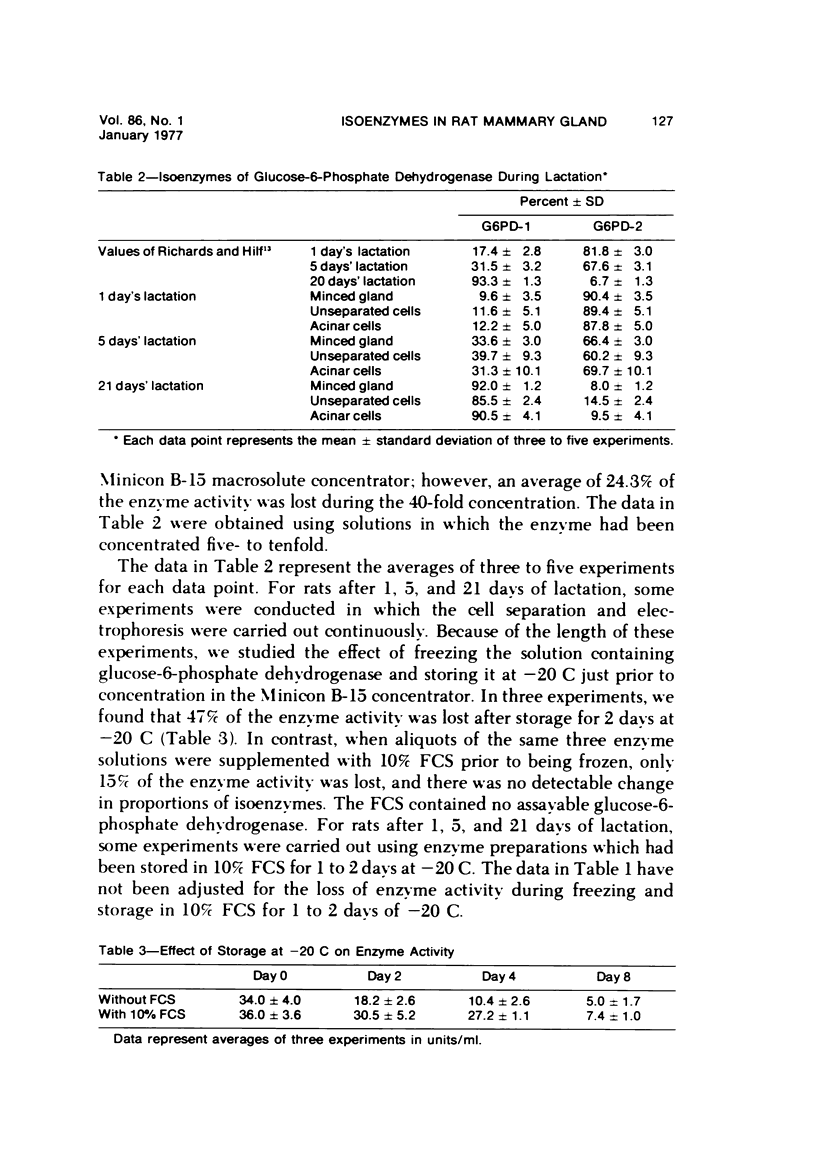
- Richards A. H., Hilf R. Influence of pregnancy, lactation and involution on glucose-6-phosphate dehydrogenase and lactate dehydrogenase in the rat mammary gland. Endocrinology. 1972 Jul;91(1):287–295. doi: 10.1210/endo-91-1-287. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B. K., Owens I. S., Topper Y. J. An early effect of prolactin on the formation of -lactalbumin by mouse mammary epithelial cells. J Biol Chem. 1973 Jan 25;248(2):467–471. [PubMed] [Google Scholar]