Abstract
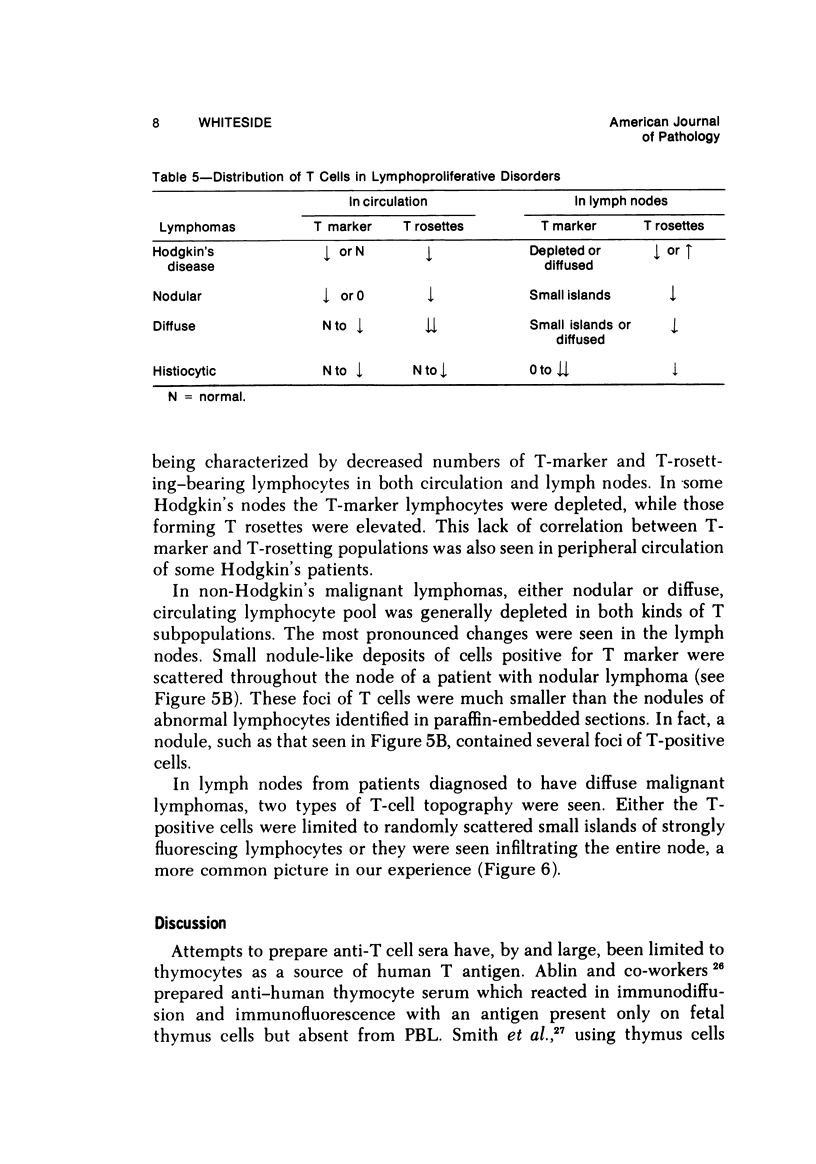
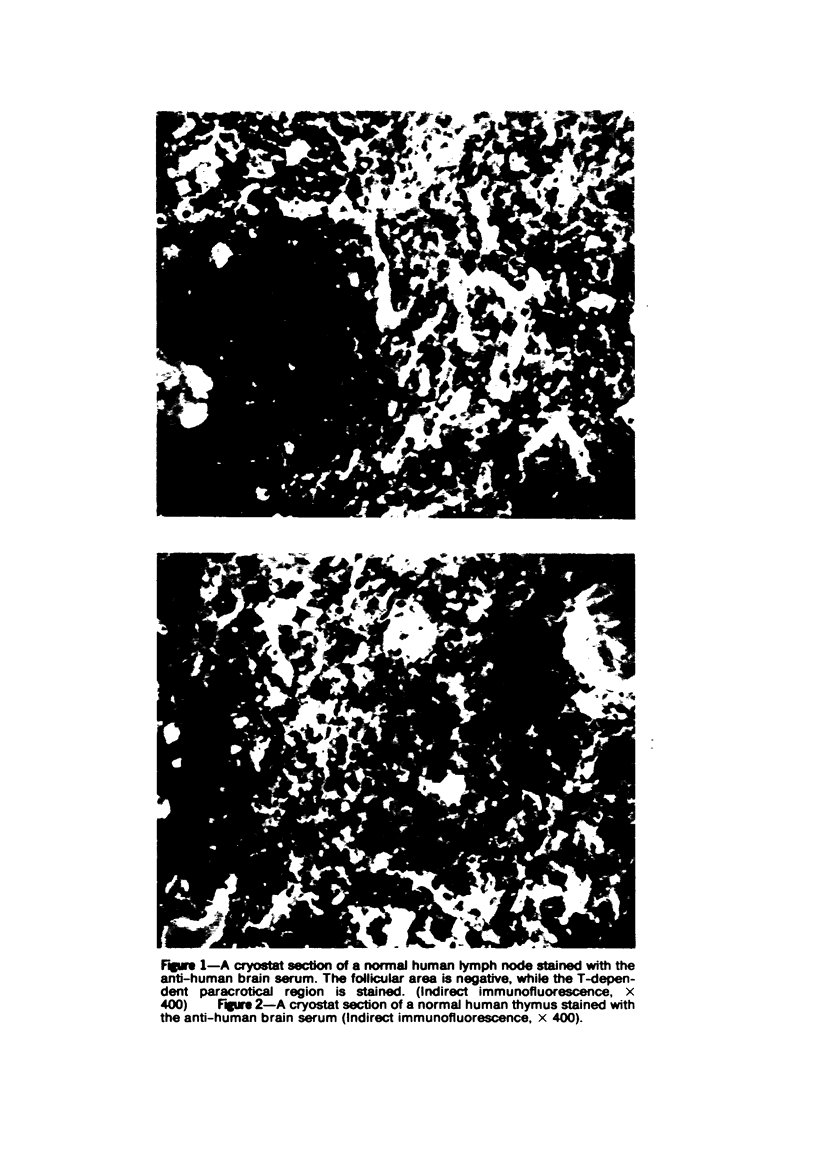
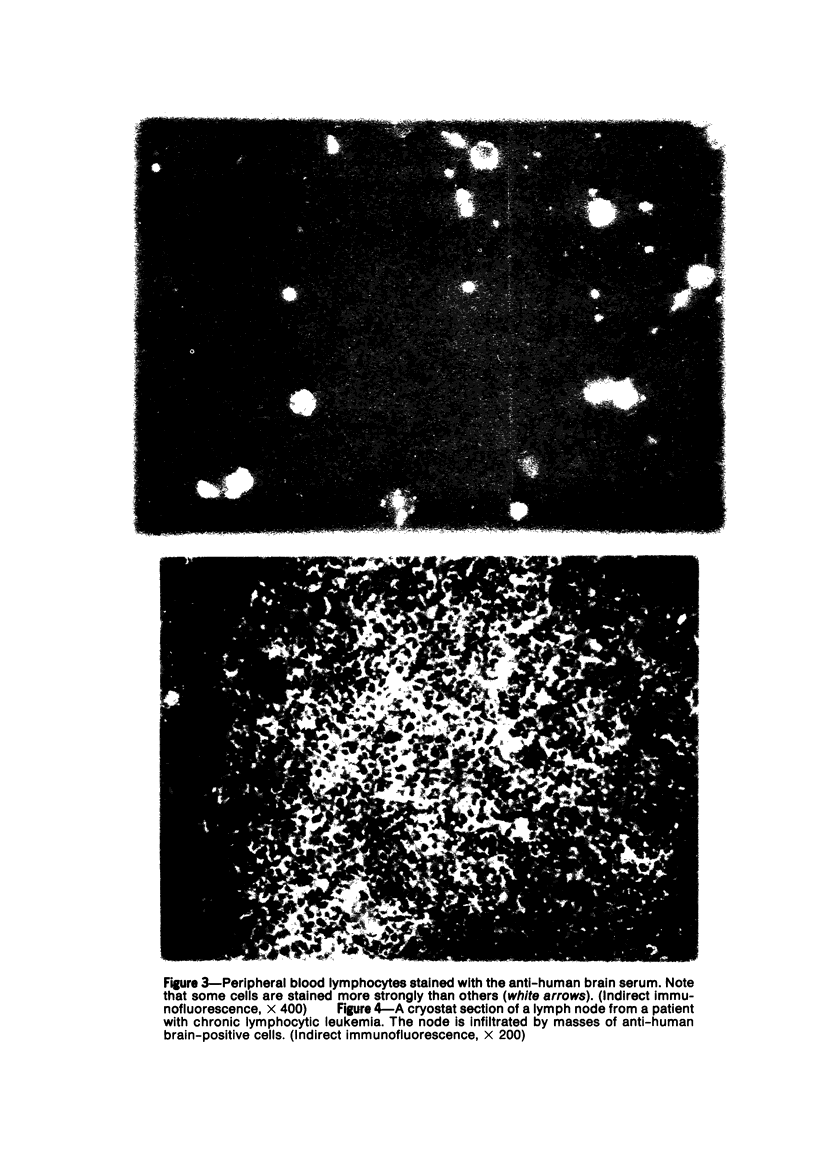
Specific anti-human T-cell serum was prepared in rabbits by multiple subcutaneous injections of human brain homogenates in incomplete Freund's adjuvant. The serum was exhaustively absorbed with human RBCs, lyophilized human liver, lyophilized normal human serum, and peripheral blood lymphocytes from patients with chronic lymphocytic leukemia (CLL). Specificity of the antiserum for human T lymphocytes was tested by indirect immunofluorescence. It stained 70 to 80% of lymphocytes in circulation, 95% of thymus, 27 to 35% of spleen, 5 to 10% of tonsil lymphocytes, and over 90% of phytohemagglutinin-stimulated lymphocytes in vitro. Only T-dependent areas of cryostat-sectioned human lymph nodes stained with the antiserum. It did not stain circulating lymphocytes which formed HEAC rosettes, plasma cells in marrows of multiple myeloma patients or macrophages. After removal of HEAC rosettes by centrifugation in Ficoll-Hypaque, 75% of interface cells formed E rosettes and 65 to 75% stained with the antiserum. The antiserum was used in studies of lymphocytes in chronic and acute lymphocytic leukemias, lymphomas, and other lymphoproliferative diseases. Numbers and distribution in the circulation, spleen and nodes of lymphocytes bearing the T marker were significantly altered in patients with these disorders.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablin R. J., Morris A. J. Immunofluorescent identification of a human thymic specific antigen. Blut. 1974 Jul;29(1):32–36. doi: 10.1007/BF01631578. [DOI] [PubMed] [Google Scholar]
- Baxley G., Bishop G. B., Cooper A. G., Wortis H. H. Rosetting of human red blood cells to thymocytes and thymus-derived cells. Clin Exp Immunol. 1973 Nov;15(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Cantor H., Simpson E., Sato V. L., Fathman C. G., Herzenberg L. A. Characterization of subpopulations of T lymphocytes. I. Separation and functional studies of peripheral T-cells binding different amounts of fluorescent anti-Thy 1.2 (theta) antibody using a fluorescence-activated cell sorter (FACS). Cell Immunol. 1975 Jan;15(1):180–196. doi: 10.1016/0008-8749(75)90174-4. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Cellular and humoral response to transplantation antigens. I. Development of alloantibody-forming cells and cytotoxic lymphocytes in the graft-versus-host reaction. J Exp Med. 1971 Aug 1;134(2):553–564. doi: 10.1084/jem.134.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. II. Evidence for separate stimulatory and inhibitory cells. J Exp Med. 1973 Dec 1;138(6):1496–1505. doi: 10.1084/jem.138.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathman C. G., Small M., Herzenberg L. A., Weissman I. L. Thymus cell maturation. II. Differentiation of three "mature" subclasses in vivo. Cell Immunol. 1975 Jan;15(1):109–128. doi: 10.1016/0008-8749(75)90169-0. [DOI] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Autonomy of thymus-processed lymphocytes (T cells) for their education into cytotoxic cells. Eur J Immunol. 1972 Dec;2(6):498–501. doi: 10.1002/eji.1830020605. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A., Gold P., Shuster J., Colquhoun B., Freedman S. O. Ability of anti-brain heteroantisera to distinguish thymus-derived lymphocytes in various species. Clin Immunol Immunopathol. 1974 Sep;3(1):1–15. doi: 10.1016/0090-1229(74)90019-1. [DOI] [PubMed] [Google Scholar]
- Kontiainen S., Andersson L. C. Antigen-binding T cells as helper cells. Separation of helper cells by immune rosette formation. J Exp Med. 1975 Oct 1;142(4):1035–1039. doi: 10.1084/jem.142.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband E., Boyse E. A. Immunocompetent cells among mouse thymocytes: a minor population. Science. 1971 Jun 18;172(3989):1258–1260. doi: 10.1126/science.172.3989.1258. [DOI] [PubMed] [Google Scholar]
- Lueker R. D., Abdin Z. H., Williams R. C., Jr Peripheral blood T and B lymphocytes during acute rheumatic fever. J Clin Invest. 1975 May;55(5):975–985. doi: 10.1172/JCI108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
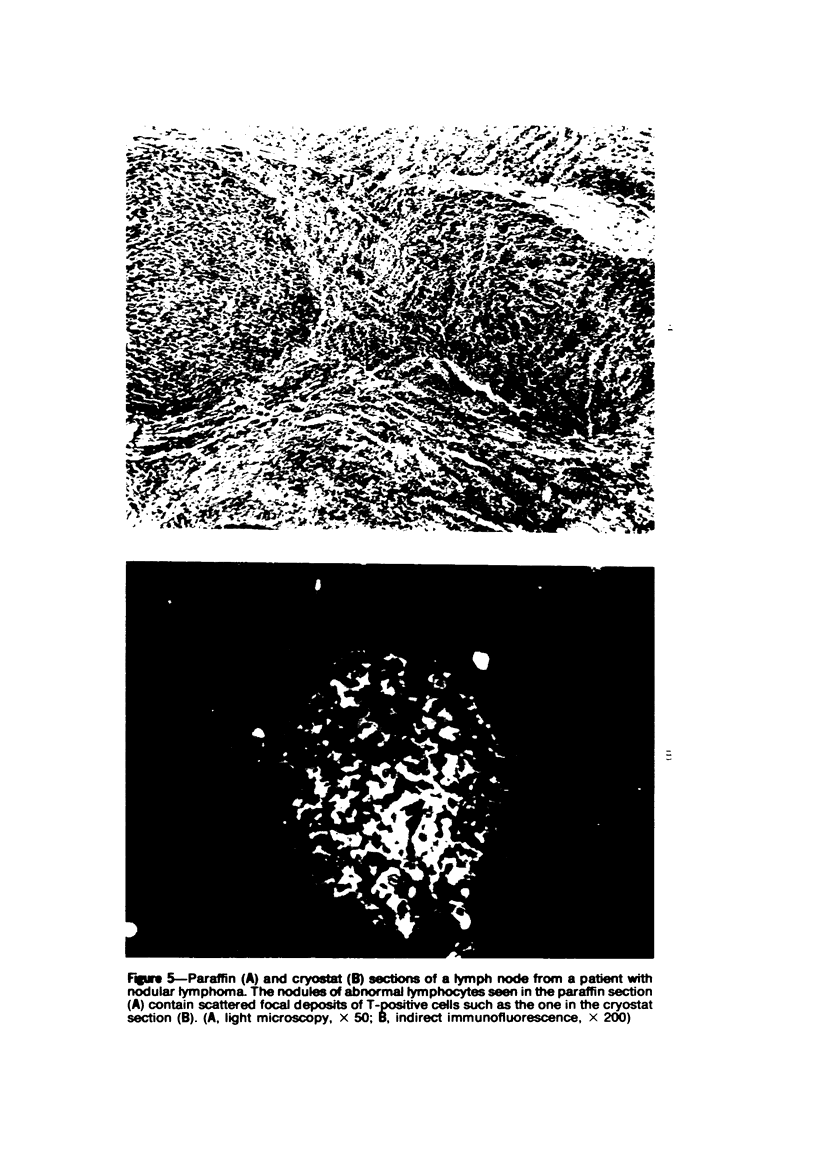
- Mendes N. F., Tolnai M. E., Silveira N. P., Gilbertsen R. B., Metzgar R. S. Technical aspects of the rosette tests used to detect human complement receptor (B) and sheep erythrocyte-binding (T) lymphocytes. J Immunol. 1973 Sep;111(3):860–867. [PubMed] [Google Scholar]
- Owen F. L., Fanger M. W. Studies on the human T-lymphocyte population. II. The use of a T-cell specific antibody in the partial isolation and characterization of the human lymphocyte receptor for sheep red blood cells. J Immunol. 1974 Oct;113(4):1138–1144. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
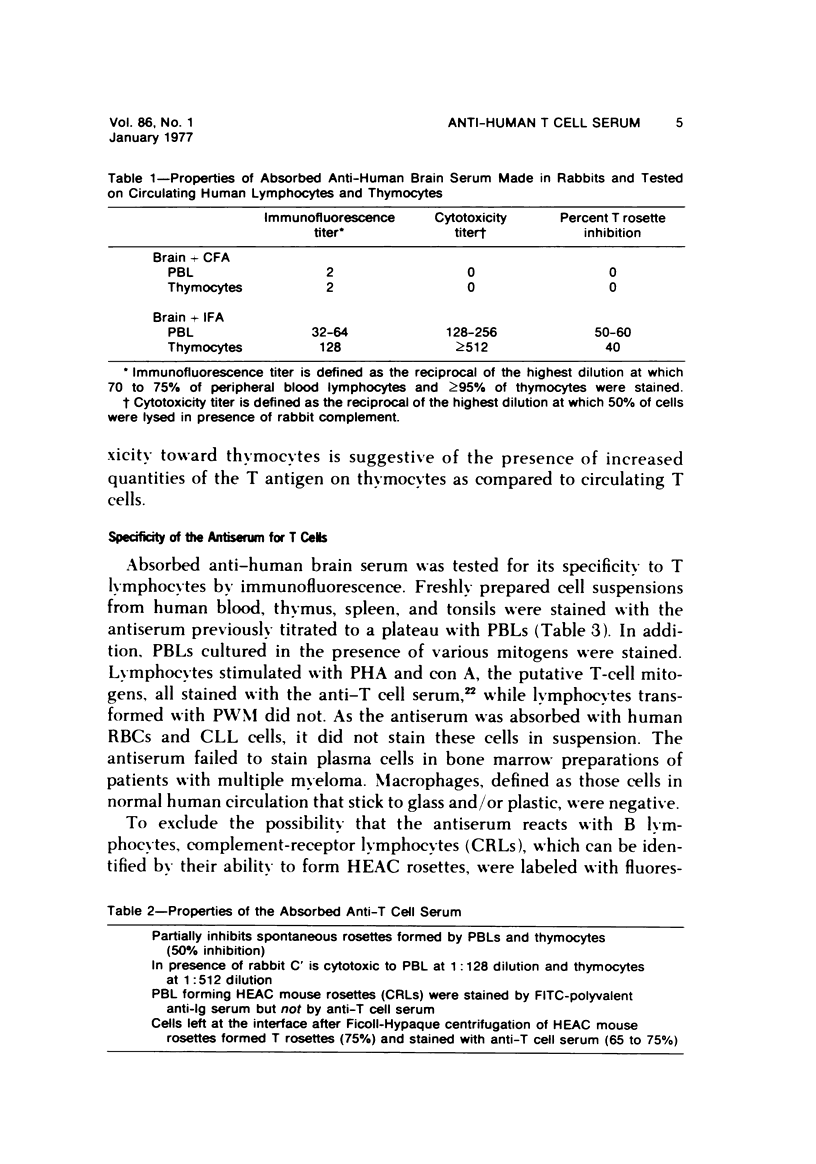
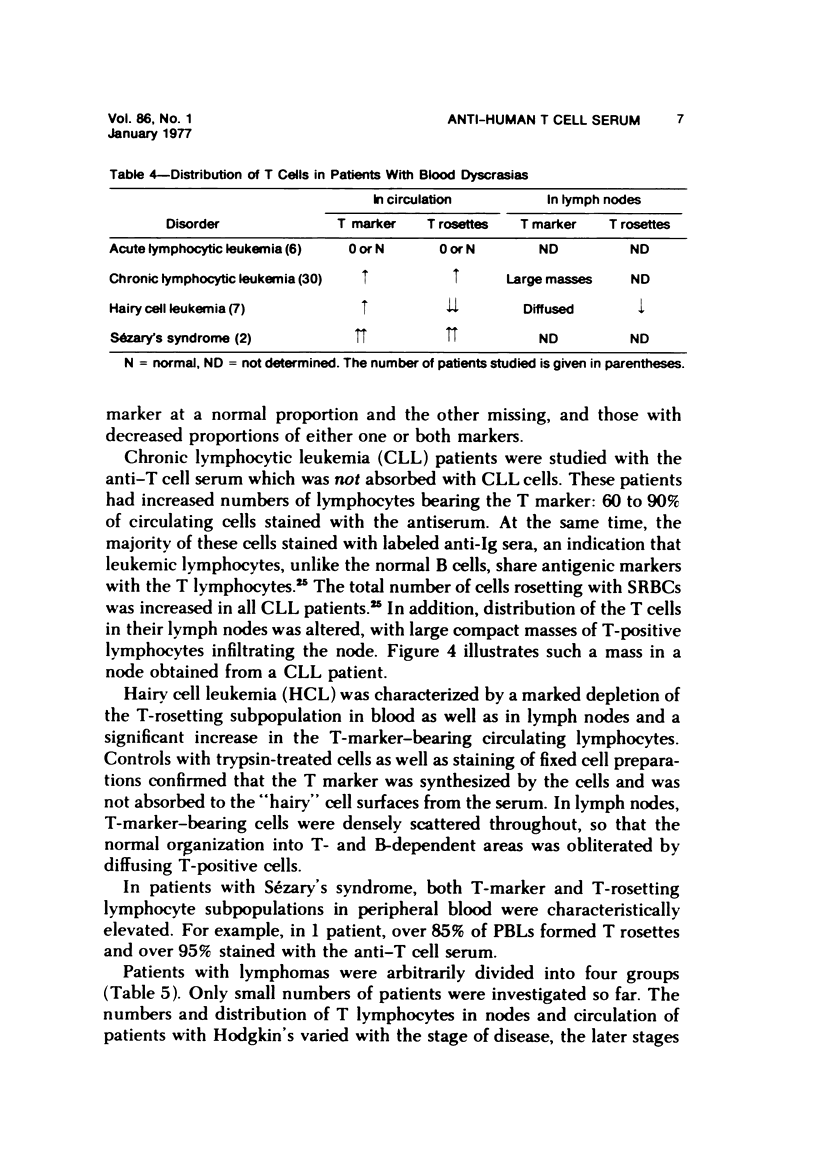
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Rydén A., Hägg L. B., Loor F. Active synthesis of immunoglobulin receptors for antigen by T lymphocytes. Nature. 1974 Jan 11;247(5436):106–108. doi: 10.1038/247106a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Galili U. Antigenic differences between T and B lymphocytes in man. Isr J Med Sci. 1974 Jul;10(7):715–724. [PubMed] [Google Scholar]
- Smith R. W., Terry W. D., Buell D. N., Sell K. W. An antigenic marker for human thymic lymphocytes. J Immunol. 1973 Mar;110(3):884–887. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes: II. Mitogen responsiveness of T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Nov 1;142(5):1041–1051. doi: 10.1084/jem.142.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Takada Y., Ito U., Minowada J. Shared antigenic determinants between human brain and human T-cell line. Clin Exp Immunol. 1974 Dec;18(4):491–498. [PMC free article] [PubMed] [Google Scholar]
- Thiele H. G., Stark R., Keeser D., Zimpel H. Letter: Lack of antigenic correlations between brain and thymus. Lancet. 1973 Dec 22;2(7843):1447–1447. doi: 10.1016/s0140-6736(73)92845-6. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Berardi R. S., Rabin B. S. Quantitation of human peripheral blood T and B lymphocytes. Int Arch Allergy Appl Immunol. 1975;48(6):731–738. doi: 10.1159/000231361. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Rabin B. S. Surface immunoglobulin on activated human peripheral blood thymus-derived cells. J Clin Invest. 1976 Mar;57(3):762–771. doi: 10.1172/JCI108335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody J. N., Ahmed A., Knudsen R. C., Strong D. M., Sell K. W. Human T-cell heterogeneity as delineated with a specific human thymus lymphocyte antiserum. In vitro effects on mitogen response mixed leukocyte culture, cell-mediated lymphocytotoxicity, and lymphokine production. J Clin Invest. 1975 May;55(5):956–966. doi: 10.1172/JCI108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]