Abstract
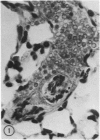
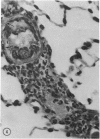
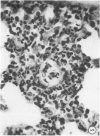
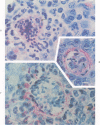
When mice are challenged intravenously with schistosomula of Schistosoma mansoni, host cell reaction and parasite attrition proceed entirely in the lung, where these events can be followed by quantitative histology and worm recovery. In nonimmune animals the destruction of schistosomula in the lungs proceeds gradually, resulting in the elimination of about 80% of the challenge organisms after 6 days. Cell reaction begins promptly, as evidenced by the appearance of neutrophilic foci around many of the lung schistosomula within 30 minutes after injection, and results in increasing numbers of damaged organisms and residual inflammatory foci 24 hours and 6 days later, respectively. In contrast, when schistosomula are injected into mice immune by virtue of an established S. mansoni infection, parasite destruction is augmented and accelerated, a process already evident by 24 hours. By the sixth day, 98% of the challenge organisms have been eliminated, a substantially greater reduction in parasite survival than that occurring in the normal host. This increased attrition of schistosomula is also reflected in the decreased numbers of parasites recovered from minced lung tissue of immune mice 6 days after challenge. Immune cellular inflammatory reactions to schistosomula are, likewise, greatly intensified and can be readily distinguished from those of normal mice by the proportions of parasites involved and by the large numbers of eosinophils surrounding them. In some instances, degranulation of eosinophils onto the parasite tegument is observed. Schistosomula cultured for 24 or 44 hours in a medium containing mouse red blood cells elicit significantly less cellular reaction and show greater survival in the lungs of immune animals than do freshly derived schistosomula. It would therefore appear that the susceptibility of maturing schistosomes to immune cellular attack is limited to the first day or two after their metamorphosis from cercariae. These observations form the framework of a new in vivo model for analyzing the dynamics of the cellular and humoral processes involved in the immune destruction of a metazoan parasite. The model also lends itself to studies of the immunologic interrelationships between innate and acquired resistance to infection with schistosomes, as well as the mechanisms by which these parasites evade the host immune response.
Full text
PDF
















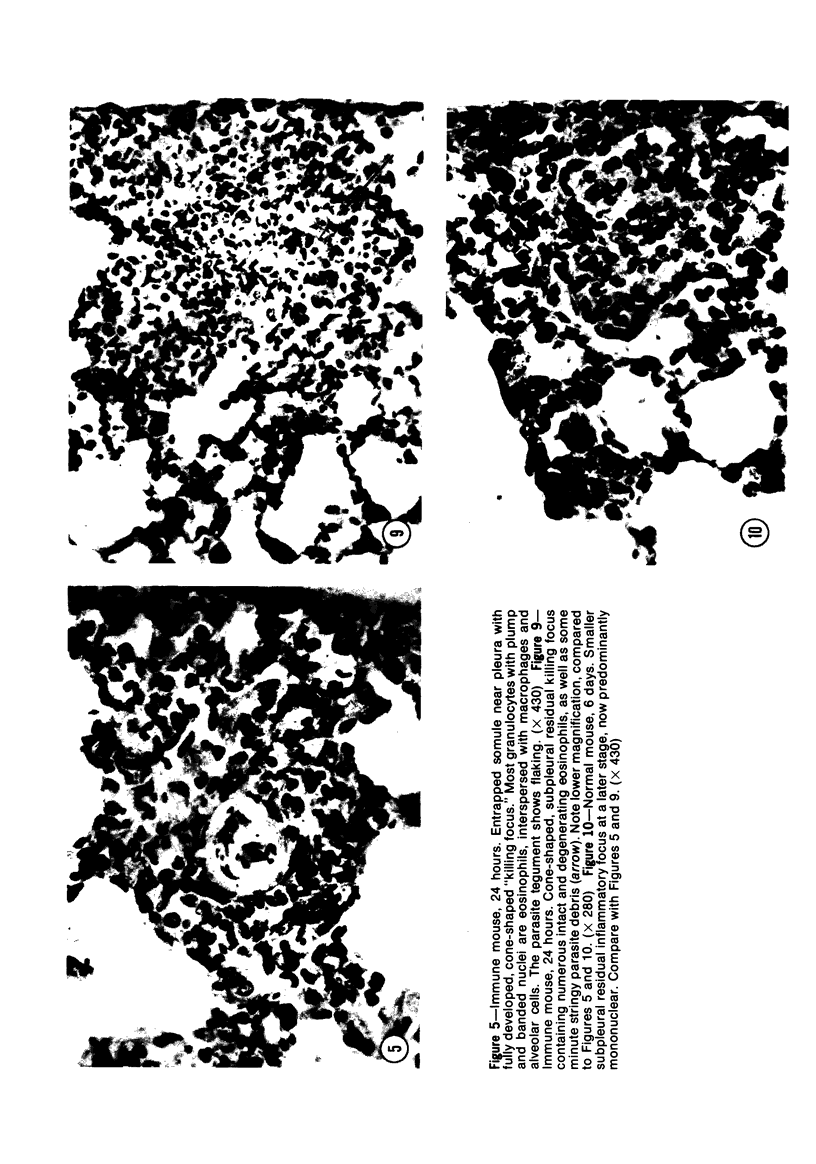

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth A. E., Sturrock R. F., Houba V., Rees P. H. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature. 1974 Dec 6;252(5483):503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. A., Smithers S. R. The effects of immune rhesus monkey serum on schistosomula of Schistosoma mansoni during cultivation in vitro. Int J Parasitol. 1972 Mar;2(1):79–98. doi: 10.1016/0020-7519(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Goldring O. L., Clegg J. A., Smithers S. R., Terry R. J. Acquisition of human blood group antigens by Schistosoma mansoni. Clin Exp Immunol. 1976 Oct;26(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Hsü S. Y., Hsü H. F., Penick G. D., Lust G. L., Osborne J. W., Cheng H. F. Mechanism of immunity of schistosomiasis: histopathologic study of lesions elicited in rhesus monkeys during immunizations and challenge with cercariae of Schistosoma japonicum. J Reticuloendothel Soc. 1975 Sep;18(3):167–185. [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg F., Sher A., Gibbons N., Doughty B. L. Eosinophil-enriched inflammatory response to schistosomula in the skin of mice immune to Schistosoma mansoni. Am J Pathol. 1976 Sep;84(3):479–500. [PMC free article] [PubMed] [Google Scholar]
- Machado A. J., Gazzinelli G., Pellegrino J., Dias de Silva W. Schistosoma mansoni: the role of the complement C3-activating system in the cercaricidal action of normal serum. Exp Parasitol. 1975 Aug;38(1):20–29. doi: 10.1016/0014-4894(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell K. D., Dean D. A., Stafford E. E. Resistance to infection with Schistosoma mansoni after immunization with worm extracts or live cercariae: role of cytotoxic antibody in mice and guinea pigs. Am J Trop Med Hyg. 1975 Nov;24(6 Pt 1):955–962. doi: 10.4269/ajtmh.1975.24.955. [DOI] [PubMed] [Google Scholar]
- Sher A., Mackenzie P., Smithers S. R. Decreased recovery of invading parasites from the lungs as a parameter of acquired immunity to schistosomiasis in the mouse. J Infect Dis. 1974 Dec;130(6):626–633. doi: 10.1093/infdis/130.6.626. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The immunology of schistosomiasis. Adv Parasitol. 1969;7:41–93. doi: 10.1016/s0065-308x(08)60434-0. [DOI] [PubMed] [Google Scholar]
- VON LICHTENBERG F., SADUN E. H., BRUCE J. I. Tissue responses and mechanisms of resistance in schistosomiasis mansoni in abnormal hosts. Am J Trop Med Hyg. 1962 May;11:347–356. doi: 10.4269/ajtmh.1962.11.347. [DOI] [PubMed] [Google Scholar]
- von LICHTENBERG, RITCHIE L. S. Cellular resistance against schistosomula of Schistosoma mansoni in Macaca mulatta monkeys following prolonged infections. Am J Trop Med Hyg. 1961 Nov;10:859–869. doi: 10.4269/ajtmh.1961.10.859. [DOI] [PubMed] [Google Scholar]