Abstract
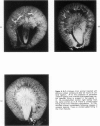
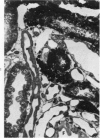
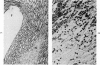
The pathogenesis of experimental unilateral hydronephrosis was studied in the rat, with emphasis on the role of medullary vascular obstruction. The medullary circulation was evaluated after increasing periods of ureteral obstruction (up to 48 hours) using in vivo perfusion of silicone rubber or colloidal carbon. Evidence of inner medullary hypoperfusion was seen after 1 hour, and by 6 hours, the entire papilla was ischemic. Papillar hypoperfusion was still present at 24 and 48 hours, but significant recirculation occurred in the presence of continued obstruction. Release of ureteral obstruction caused rapid reversal of the perfusion defect. Histologic studies of the renal parenchyma adjacent to the pelvis showed increasing vascular congestion, focal interstitial edema, and extravasation of erythrocytes; these changes were also diminished after release of obstruction. Endothelial swelling or thrombosis in inner medullary blood vessels was not observed. Inner medullary tubules showed degenerative changes in kidneys that had been obstructed for 6 hours or more; at later periods (18 hours or more) focal necrosis was seen, but it never involved the entire papilla. Thus, reversible collapse of inner medullary blood vessels occurs in acute unilateral hydronephrosis and may provide the basis for the development of ischemic tubular damage. The findings suggest that the vascular obstruction may be due to increased intrapelvic pressure, rather than endothelial swelling or thrombosis. The vascular-tubular defect may be relevant to the altered medullary physiology observed in this condition.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. D., Bulger R. E., Trump B. F. The ultrastructure of the functioning kidney. Lab Invest. 1967 Feb;16(2):220–246. [PubMed] [Google Scholar]
- Harris R. H., Yarger W. E. Renal function after release of unilateral ureteral obstruction in rats. Am J Physiol. 1974 Oct;227(4):806–815. doi: 10.1152/ajplegacy.1974.227.4.806. [DOI] [PubMed] [Google Scholar]
- Honda N., Aizawa C., Morikawa A., Yoshitoshi Y. Effect of elevated ureteral pressure on renal medullary osmolal concentration in hydropenic rabbits. Am J Physiol. 1971 Sep;221(3):698–703. doi: 10.1152/ajplegacy.1971.221.3.698. [DOI] [PubMed] [Google Scholar]
- Hársing L., Szántó G., Bartha J. Renal circulation during stop flow in the dog. Am J Physiol. 1967 Oct;213(4):935–938. doi: 10.1152/ajplegacy.1967.213.4.935. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. The renal response to ureteral obstruction: a model for the study of factors which influence glomerular filtration pressure. J Lab Clin Med. 1970 Sep;76(3):373–382. [PubMed] [Google Scholar]
- MUIRHEAD E. E., VANATTA J., GROLLMAN A. Papillary necrosis of the kidney; a clinical and experimental correlation. J Am Med Assoc. 1950 Mar 4;142(9):627–631. doi: 10.1001/jama.1950.02910270017004. [DOI] [PubMed] [Google Scholar]
- McDougal W. S., Wright F. S. Defect in proximal and distal sodium transport in post-obstructive diuresis. Kidney Int. 1972 Dec;2(6):304–317. doi: 10.1038/ki.1972.114. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Bulger R. E., Cutler R. E., Jervis H. R., Benditt E. P. Unilateral obstructive nephropathy in the rabbit. I. Early morphologic, physiologic, and histochemical changes. Lab Invest. 1973 Apr;28(4):456–467. [PubMed] [Google Scholar]
- Rao N. R., Heptinstall R. H. Experimental hydronephrosis. A microangiographic study. Invest Urol. 1968 Sep;6(2):183–204. [PubMed] [Google Scholar]
- SELKURT E. E. EFFECT OF URETERAL BLOCKADE ON RENAL BLOOD FLOW AND URINARY CONCENTRATING ABILITY. Am J Physiol. 1963 Aug;205:286–292. doi: 10.1152/ajplegacy.1963.205.2.286. [DOI] [PubMed] [Google Scholar]
- SHEEHAN H. L., DAVIS J. C. Experimental hydronephrosis. AMA Arch Pathol. 1959 Aug;68(2):185–225. [PubMed] [Google Scholar]
- Strock P. E., Majno G. Vascular responses to experimental tourniquet ischemia. Surg Gynecol Obstet. 1969 Aug;129(2):309–318. [PubMed] [Google Scholar]
- Summers W. K., Jamison R. L. The no reflow phenomenon in renal ischemia. Lab Invest. 1971 Dec;25(6):635–643. [PubMed] [Google Scholar]
- Vaughan E. D., Jr, Sorenson E. J., Gillenwater J. Y. Effects of acute and chronic ureteral obstruction on renal hemodynamics and function. Surg Forum. 1968;19:536–538. [PubMed] [Google Scholar]