Abstract
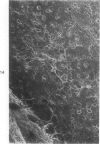
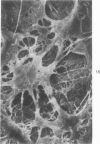
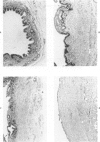
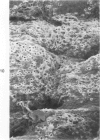
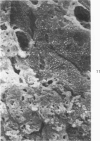
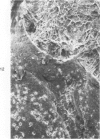
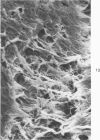
Common biliary ducts of 32 adult mongrel dogs were ligated for a period of 2 days to 6 weeks. Sham-operated animals served as controls. Bile ducts were removed at sacrifice, and biophysical, morphologic, and biochemical parameters were measured. Our study shows that biliary duct ligation results in an immediate increase of intraductular pressure and is followed quickly by significant increase in the rate of collagen synthesis and the activity of prolyl hydroxylase. Histologic data show subepithelial inflammation followed by marked increases in periductular fibrosis. This fibroproliferative response is paralleled by peak levels of prolyl hydroxylase activity at 2 weeks prostligation. Paradoxically, bile ducts continuously distend throughout the ligation period despite increased fibroplasia. We present here the first topographic (SEM) study of normal and ligated common bile duct epithelium. Following 2 weeks of ligation large crater-like fenestrae are seen ductular epithelial surfaces. This is followed by focal epithelial sloughing. We speculate that the continuous distention and epithelial necrosis seen in the present study may be due to biliary stasis and/or subepithelial infiltration of bile through epithelial fenestrae. This hypothesis is supported by our studies which show that collagen extractibility is markedly increased by the addition of bile to the homogenate.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Dalferes E. R., Jr Macromolecules in the arterial wall in relation to injury and repair--a survey. Angiology. 1974 Nov;25(10):649–681. doi: 10.1177/000331977402501006. [DOI] [PubMed] [Google Scholar]
- Bondjers G., Björnheden T. Experimental atherosclerosis induced by mechanical trauma in rats. Atherosclerosis. 1970 Sep-Oct;12(2):301–306. doi: 10.1016/0021-9150(70)90109-7. [DOI] [PubMed] [Google Scholar]
- Compagno J., Grisham J. W. Scanning electron microscopy of extrahepatic biliary obstruction. Arch Pathol. 1974 Jun;97(6):348–351. [PubMed] [Google Scholar]
- FITCH S. M., HARKNESS M. L., HARKNESS R. D. Extraction of collagen from tissues. Nature. 1955 Jul 23;176(4473):163–163. doi: 10.1038/176163a0. [DOI] [PubMed] [Google Scholar]
- Hauss W. H. Uber die Rolle des Mesenchums in der Genese der Arteriosklerose. Virchows Arch A Pathol Pathol Anat. 1973 Apr 19;359(2):135–156. [PubMed] [Google Scholar]
- Helin P., Lorenzen I., Garbarsch C., Matthiessen M. E. Repair in arterial tissue. Morphological and biochemical changes in rabbit aorta after a single dilatation injury. Circ Res. 1971 Nov;29(5):542–554. doi: 10.1161/01.res.29.5.542. [DOI] [PubMed] [Google Scholar]
- Koyama K., Muto I., Yamauchi H., Takagi Y., Anezaki T. Biochemical study of fibrosis in the rat liver in biliary obstruction. Tohoku J Exp Med. 1975 Jun;116(2):161–172. doi: 10.1620/tjem.116.161. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mueller J. C., Jones A. L., Long J. A. Topographic and subcellular anatomy of the guinea pig gallbladder. Gastroenterology. 1972 Nov;63(5):856–868. [PubMed] [Google Scholar]
- Shimamoto T. Hyperreactive arterial endothelial cells in atherogenesis and cyclic AMP phosphodiesterase inhibitor in prevention and treatment of atherosclerotic disorders. Jpn Heart J. 1975 Jan;16(1):76–97. doi: 10.1536/ihj.16.76. [DOI] [PubMed] [Google Scholar]
- Uitto J. A method for studying collagen biosynthesis in human skin biopsies in vitro. Biochim Biophys Acta. 1970 Mar 24;201(3):438–445. doi: 10.1016/0304-4165(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Goldfischer S., Schiller B., Kasak L. E. Modification of the effects of hypertension on lysosomes and connective tissue in the rat aorta. Circ Res. 1974 Feb;34(2):233–241. doi: 10.1161/01.res.34.2.233. [DOI] [PubMed] [Google Scholar]
- Zaki F. G. Ultrastructure of hepatic cholestasis. Medicine (Baltimore) 1966 Nov;45(6):537–545. doi: 10.1097/00005792-196645060-00019. [DOI] [PubMed] [Google Scholar]