Abstract
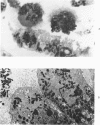
Rat kidneys were disaggregated with 0.25% trypsin. Cell were separated by velocity sedimentation in a previously described isokinetic gradient, by isopycnic sedimentation, and by velocity sedimentation followed by isopycnic sedimetation. In some fractions from the isokinetic gradient, 71.8+/-2.4+ of the nucleated cells contained histochemically demonstrable alkaline phosphatase (HDAP); in semithin sections, 62.7+/-2.3% of these cells had brush borders. The correspondence between fractions enriched for cells with HDAP and fractions enriched for brush border suggested that HDAP might be a suitable marker for rat proximal tubule cells. These cell constituted 46.5+/-2.6% of the nucleated cells in the starting sample suspension of kidney cells, and 81.9+/-2.7% of nucleated cells in the purified fractions from the gradients. More than 98% of nucleated cells in these fractions excluded typan blue. Following isopycnin centrifugation, the purest fractions contained 87.3+/-1.5% nucleated cells with HDAP, 9.6+/-2.5% nucleated cells iwithout HDAP, and 3.1+/-2.5% red blood cells. These proximal tubule cells had densities of 1.036 to 1.052 g/ml. With rate-zonal separation followed by isopycnic separation, the purest gradient fraction contained 93.0+/-1.9% nucleated cell with HDAP, 6.0+/-2.3% nucleated cells with HDAP, and 1.0+/-0.9% red blood cells. These proximal tubule cells sedimented a density of 1.041 g/ml. More than 98% of these cells excluded trypan blue.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspray D. W., Pitts A., Pretlow T. G., 2nd Separation of epithelial cells from suspensions of cells from the hamster parotid gland in an isokinetic density gradient of Ficoll in tissue culture medium. Anal Biochem. 1975 Jun;66(2):353–364. doi: 10.1016/0003-2697(75)90603-x. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical comparison of naphthol AS-phosphates for the demonstration of phosphatases. J Natl Cancer Inst. 1958 Mar;20(3):601–615. [PubMed] [Google Scholar]
- Gritzka T. L., Trump B. F. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am J Pathol. 1968 Jun;52(6):1225–1277. [PMC free article] [PubMed] [Google Scholar]
- Haskill J. S., Moore M. A. Two dimensional cell separation: comparison of embryonic and adult haemopoietic stem cells. Nature. 1970 May 30;226(5248):853–854. doi: 10.1038/226853a0. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Fedorko M. E. Isolation of intact megakaryocytes from guinea pig femoral marrow. Successful harvest made possible with inhibitions of platelet aggregation; enrichment achieved with a two-step separation technique. J Cell Biol. 1976 Apr;69(1):159–172. doi: 10.1083/jcb.69.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of mammalian cells using programmed gradient sedimentation. Exp Mol Pathol. 1969 Oct;11(2):139–152. doi: 10.1016/0014-4800(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Cassady I. M. Separation of mast cells in successive stages of differentiation using programmed gradient sedimentation. Am J Pathol. 1970 Dec;61(3):323–340. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Jones J., Dow S. Separation of cells having histochemically demonstrable glucose-6-phosphatase from suspensions of hamster kidney cells in an isokinetic density gradient of Ficoll in tissue culture medium. Am J Pathol. 1974 Feb;74(2):275–286. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Pichichero M. E., Hyams L. Separation of lymphocytes and macrophages from suspensions of guinea pig peritonitis exudate cells using programmed gradient sedimentation. Am J Pathol. 1971 May;63(2):255–276. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, weir E. E., Zettergren J. G. Problems connected with the separation of different kinds of cells. Int Rev Exp Pathol. 1975;14:91–204. [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., Stinson A. J. Separation of megakaryocytes from rat bone marrow cells using velocity sedimentation in an isokinetic gradient ficoll in tissue culture medium. J Cell Physiol. 1976 Jul;88(3):317–322. doi: 10.1002/jcp.1040880307. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Ganote C. E., Jennings R. B. Alterations in renal cortex following ischemic injury. 3. Ultrastructure of proximal tubules after ischemia or autolysis. Lab Invest. 1972 Apr;26(4):347–363. [PubMed] [Google Scholar]
- Zettergren J. G., Luberoff D. E., Pretlow T. G., 2nd Separation of lymphocytes from disaggregated mouse malignant neoplasms by sedimentation in gradients of ficoll in tissue culture medium. J Immunol. 1973 Sep;111(3):836–840. [PubMed] [Google Scholar]