Abstract
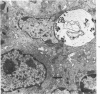
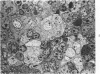
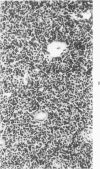
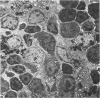
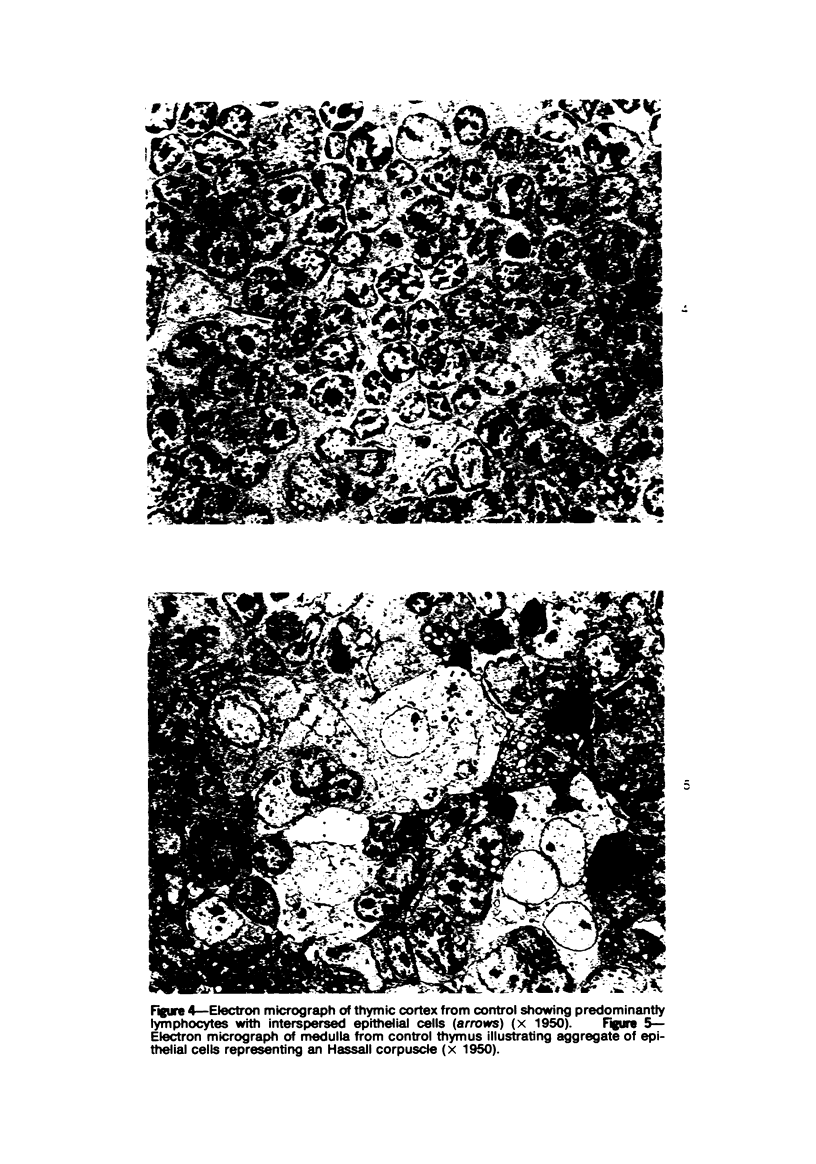
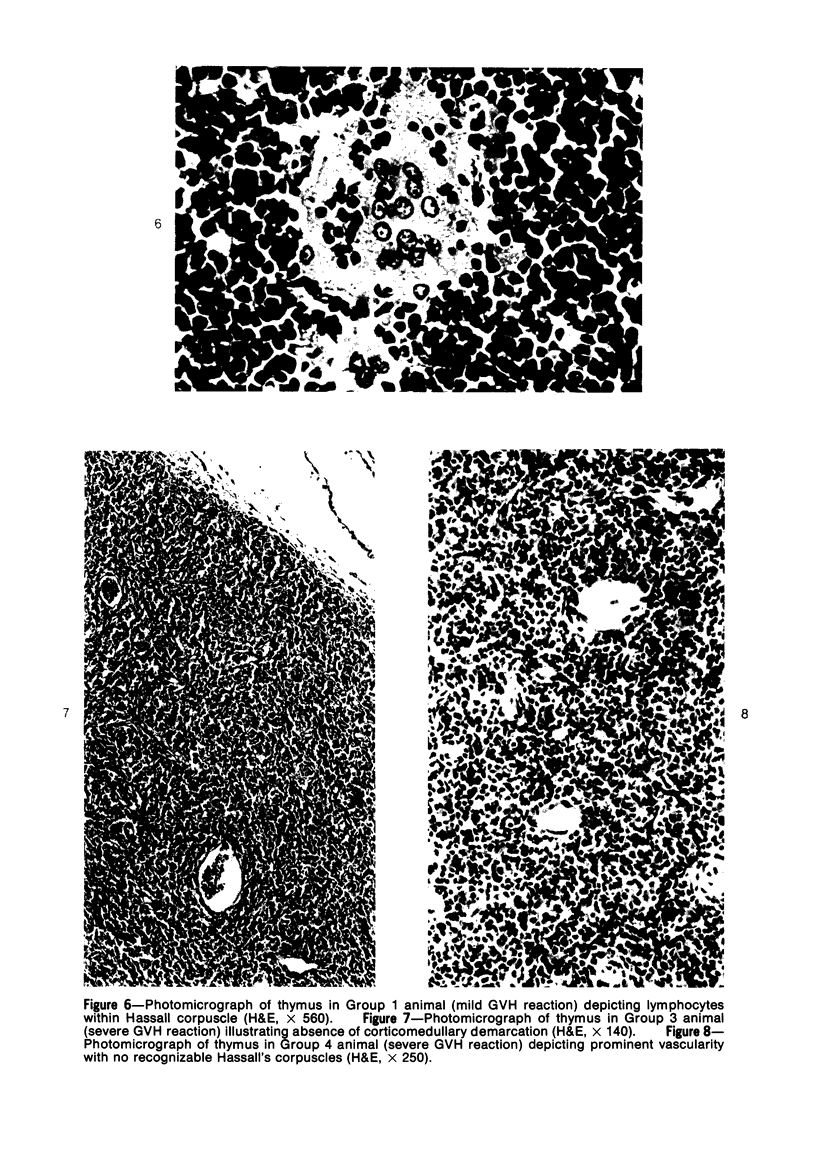
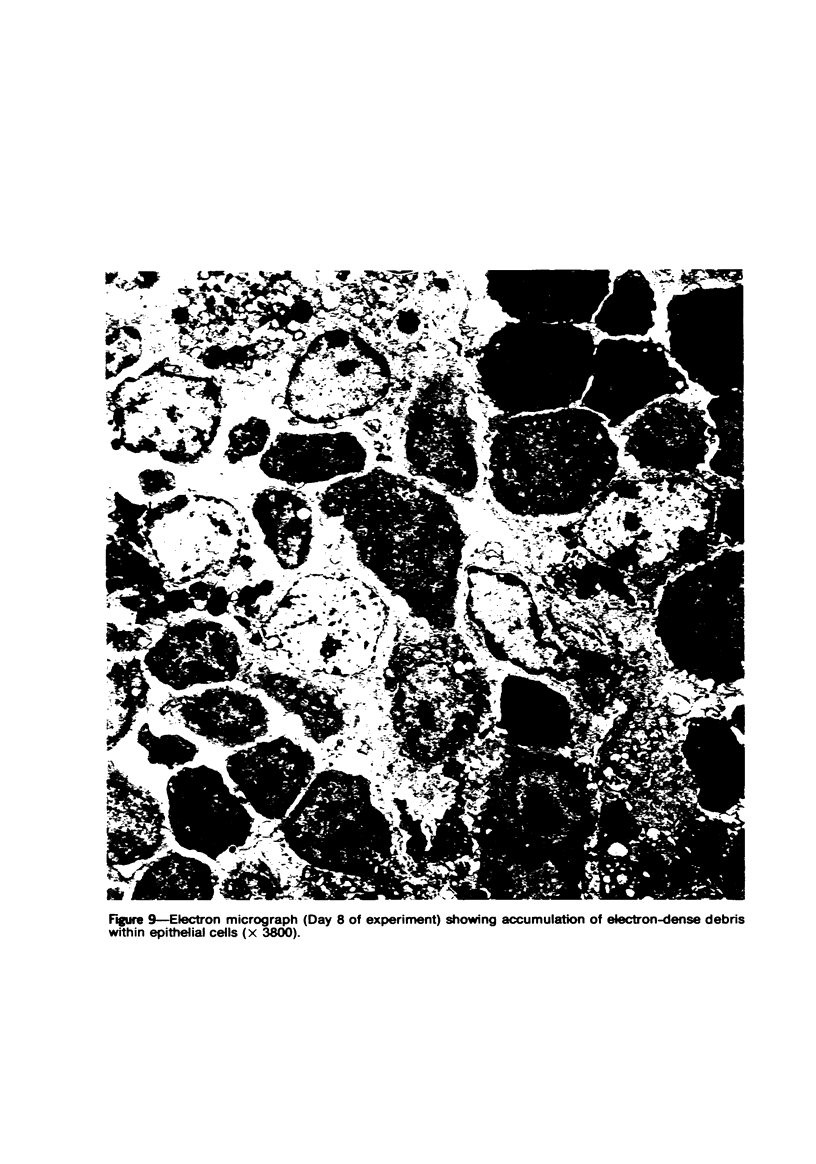
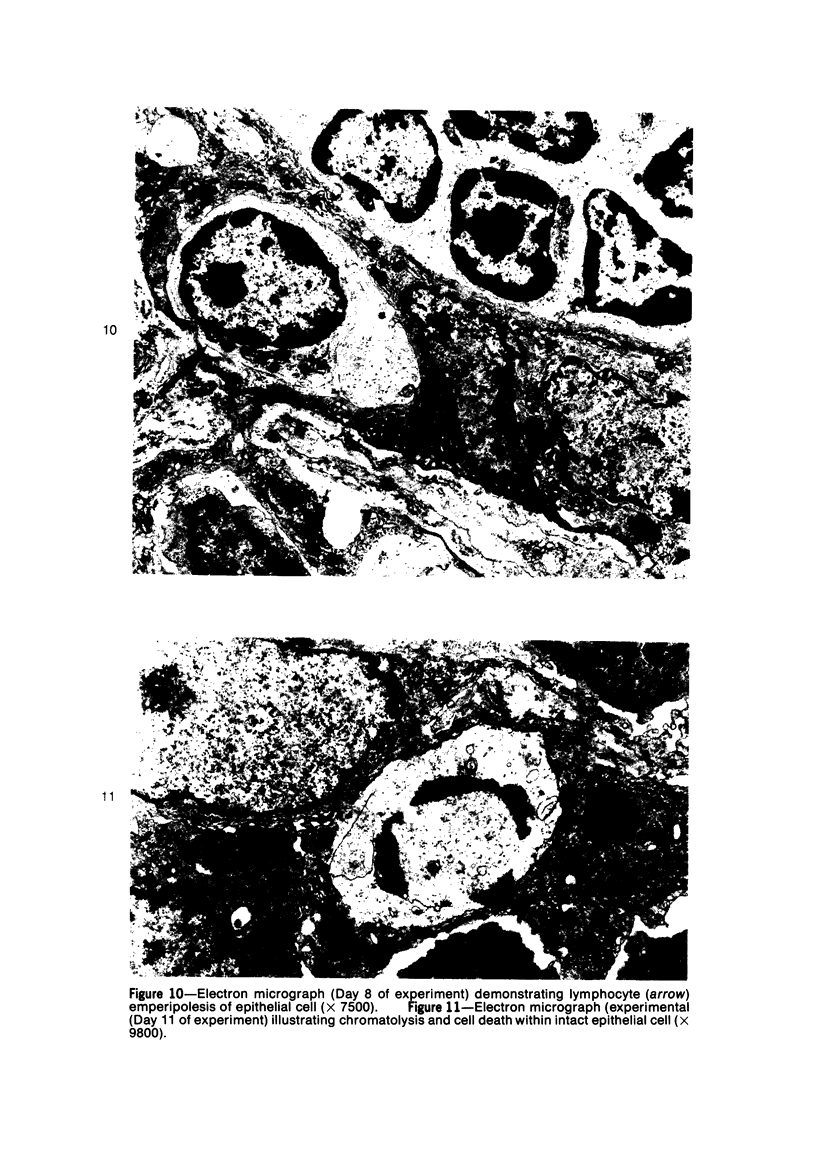
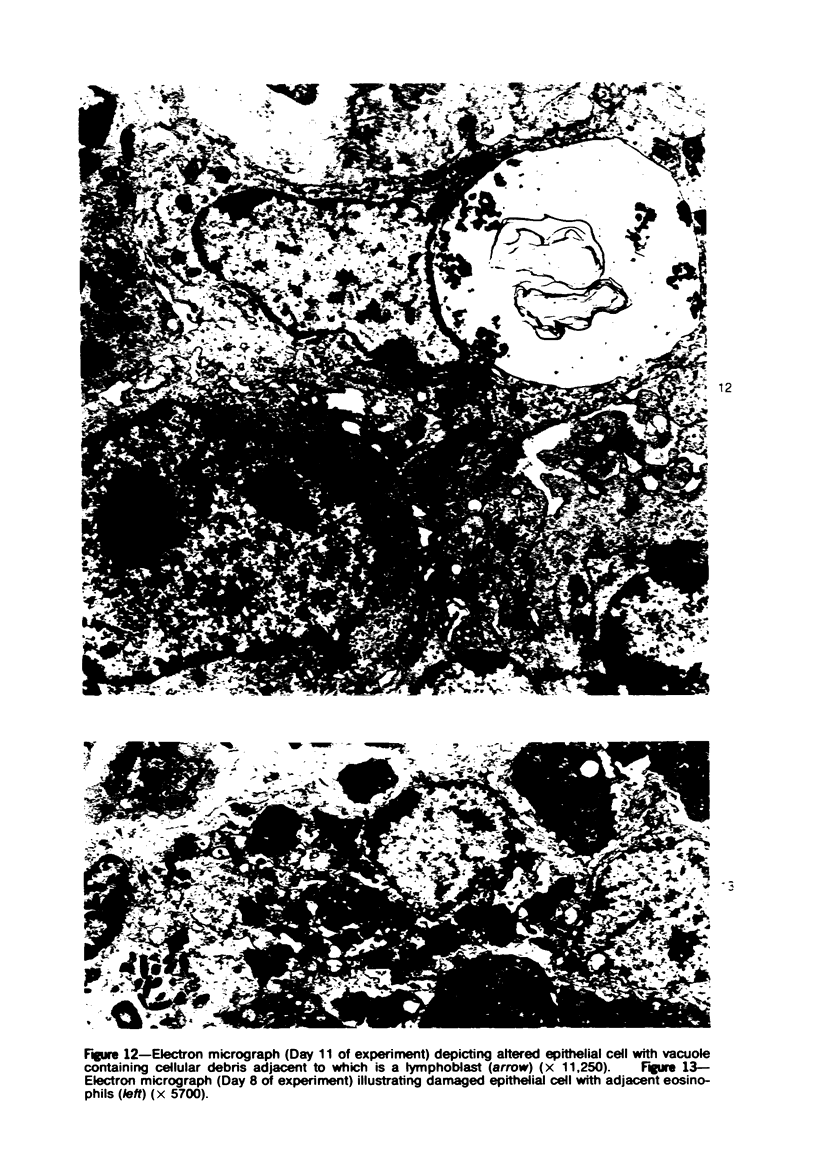
Mild, moderate, and severe graft-versus-host (GVH) reactions were induced in four series of experiments in 71 CBA X A and C57BL/6 X A F1 hybrid mice. At regular intervals post-GVH reaction induction (Days 4-42), the animals were sacrificed, autopsied, and histologically studied. Visceral alterations of GVH reaction were recorded in the spleen, lymph nodes, liver, kidney, gut, and thymus. A spectrum of thymic changes was documented, ranging from obliteration of a definable cortex and medulla with loss of Hassall's corpuscles to marked involution with complete disappearance of the gland. Ultrastructural studies revealed damage to both lymphocytes and epithelial cells along with lymphocyte emperipolesis of epithelial cells, lymphocytolysis within epithelial cells, and accumulation of numerous autophagic vacuoles containing fragments of cellular debris within epithelial cells and histiocytes. The resemblance of these alterations to human thymic dysplasia as observed in primary immunodeficient conditions was striking. The theoretical implications of these studies for the pathogenesis of human congenital immunodeficiency states are considered.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Kawamoto Y., Furuta I., Kondo T. Early morphological changes in cortical medullary thymocytes of the rat after whole-body irradiation. I. Electron-microscope observations. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Jun;21(6):545–558. doi: 10.1080/09553007214550641. [DOI] [PubMed] [Google Scholar]
- Chen T. K., Clawson C. C., Yunis E. J., Kersey J. H. Thymic aplasia with possible graft-versus-host disease. J Pediatr. 1975 Jun;86(6):904–907. doi: 10.1016/s0022-3476(75)80223-x. [DOI] [PubMed] [Google Scholar]
- Elie R., Lapp W. S. Graft-versus-host induced immunosuppression: depressed T cell helper function in vitro. Cell Immunol. 1976 Jan;21(1):31–39. doi: 10.1016/0008-8749(76)90324-5. [DOI] [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- Githens J. H., Muschenheim F., Fulginiti V. A., Robinson A., Kay H. E. Thymic alymphoplasia with XX-XY lymphoid chimerism secondary to probable maternal-fetal transfusion. J Pediatr. 1969 Jul;75(1):87–94. doi: 10.1016/s0022-3476(69)80105-8. [DOI] [PubMed] [Google Scholar]
- Grebe S. C., Streilein J. W. Graft-versus-Host reactions: a review. Adv Immunol. 1976;22:119–221. doi: 10.1016/s0065-2776(08)60549-0. [DOI] [PubMed] [Google Scholar]
- Grogan T. M., Broughton D. D., Doyle W. F. Graft-versus-host reaction (GVHR). A case report suggesting GVHR occurred as a result of maternofetal cell transfer. Arch Pathol. 1975 Jun;99(6):330–334. [PubMed] [Google Scholar]
- Grushka M., Lapp W. S. Effect of lymphoid tissue transplants on the intensity of an existing graft-versus-host reaction. Transplantation. 1974 Feb;17(2):157–163. doi: 10.1097/00007890-197402000-00001. [DOI] [PubMed] [Google Scholar]
- Kadowaki J., Thompson R. I., Zuelzer W. W., Woolley P. V., Jr, Brough A. J., Gruber D. XX-XY lymphoid chimaerism in congenital immunological deficiency syndrome with thymic alymphoplasia. Lancet. 1965 Dec 4;2(7423):1152–1156. doi: 10.1016/s0140-6736(65)92559-6. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., Meuwissen H. J., Good R. A. Graft versus host reactions following transplantation of allogeneic hematopoietic cells. Hum Pathol. 1971 Sep;2(3):389–402. doi: 10.1016/s0046-8177(71)80006-0. [DOI] [PubMed] [Google Scholar]
- Lapp W. S., Möller G. Prolonged survival of H-2 incompatible skin allografts on F1 animals treated with parental lymphoid cells. Immunology. 1969 Sep;17(3):339–344. [PMC free article] [PubMed] [Google Scholar]
- Lapp W. S., Wechsler A., Kongshavn P. A. Immune restoration of mice immunosuppressed by a graft-vs.-host reaction. Cell Immunol. 1974 Mar 30;11(1-3):419–426. doi: 10.1016/0008-8749(74)90040-9. [DOI] [PubMed] [Google Scholar]
- Möller G. Suppressive effect of graft versus host reactions on the immune response to heterologous red cells. Immunology. 1971 Apr;20(4):597–609. [PMC free article] [PubMed] [Google Scholar]
- Naiman J. L., Punnett H. H., Lischner H. W., Destiné M. L., Arey J. B. Possible graft-versus-host reaction after intrauterine transfusion for Rh erythroblastosis fetalis. N Engl J Med. 1969 Sep 25;281(13):697–701. doi: 10.1056/NEJM196909252811303. [DOI] [PubMed] [Google Scholar]
- Parkman R., Mosier D., Umansky I., Cochran W., Carpenter C. B., Rosen F. S. Graft-versus-host disease after intrauterine and exchange transfusions for hemolytic disease of the newborn. N Engl J Med. 1974 Feb 14;290(7):359–363. doi: 10.1056/NEJM197402142900703. [DOI] [PubMed] [Google Scholar]
- Parthenais E., Elie R., Lapp W. S. Soluble factors and the immune response: in vitro studies of the immunosuppression induced by the graft-versus-host reaction. Cell Immunol. 1974 Jul;13(1):164–168. doi: 10.1016/0008-8749(74)90235-4. [DOI] [PubMed] [Google Scholar]
- Shapiro M. Familial autohemolytic anemia and runting syndrome with Rh-o-specific autoantibody. Transfusion. 1967 Jul-Aug;7(4):281–296. doi: 10.1111/j.1537-2995.1967.tb05519.x. [DOI] [PubMed] [Google Scholar]
- Swasdikul D., Block M. Effect of radiation upon the "embryonic" thymus. Radiat Res. 1972 Apr;50(1):73–84. [PubMed] [Google Scholar]
- TROWELL O. A. Radiosensitivity of the cortical and medullary lymphocytes in the thymus. Int J Radiat Biol Relat Stud Phys Chem Med. 1961 Nov;4:163–173. doi: 10.1080/09553006114551091. [DOI] [PubMed] [Google Scholar]
- Weiss L., Aisenberg A. C. An electron microscope study of lymphatic tissue in runt disease. J Cell Biol. 1965 Jun;25(3 Suppl):149–177. doi: 10.1083/jcb.25.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. M., Butcher W. I., Hellerstein L. J. Early secondary disease in the rhesus monkey. II. Electron microscopy of changes in mucous membranes and external epithelia as demonstrated in the tongue and lip. Lab Invest. 1972 Jul;27(1):85–98. [PubMed] [Google Scholar]
- van Haelst U. Light and electron microscopic study of the normal and pathological thymus of the rat. II. The acute thymic involution. Z Zellforsch Mikrosk Anat. 1967;80(2):153–182. doi: 10.1007/BF00337454. [DOI] [PubMed] [Google Scholar]