Abstract
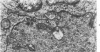
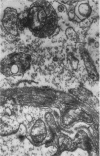
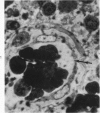
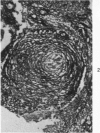
The fine structure of small blood vessels in and around ten brain tuberculomas was examined. In the peripheral reactive zone of the tuberculomas, examination of 1-μ-thick survey sections established the chronic inflammatory process and the vasculitis characterized by infiltration of the vasomurium (vessel wall) by large and small mononuclear cells. This reaction was typical of chronic epithelioid cell granuloma. Electron microscopic examination of the reactive zone confirmed the vascular proliferation and vasculitis, the venule being the most frequently involved type of blood vessel. It showed the infiltrating cells to lie amidst osmiophilic, concentrically proliferated basement membrane laminae, which formed the greater part of the thickened vessel wall, generally surrounding the endothelial cells directly, the pericytes having disappeared. This appearance, together with the results of Gomori's reticulin stain on paraffin sections, suggested that the altered basement membrane material was reticulin. The possibility is discussed that the altered basement membrane material could be antigenic and that it might be responsible for perpetuating the necrotic vascular and perivascular reaction in tuberculous meningitis and tuberculomas. The above change in the basement membrane was not encountered in the blood vessels of the surrounding edematous brain. The endothelial cells and tight junctions were relatively well-preserved. Intact arterioles could be recognized even in severely edematous brain tissue. At both sites the fine structure of the blood vessels was typical of that expected in the central nervous system. Fenestrated vessels were not seen. The perivascular astrocytic end-feet were destroyed in the reactive zone and either distended or ruptured in the overtly edematous brain tissue also. In the central caseous part of the tuberculoma, there were few blood vessels, and they were in a state of advanced necrosis, but ghost outlines of proliferated basement membrane could be seen.
Full text
PDF











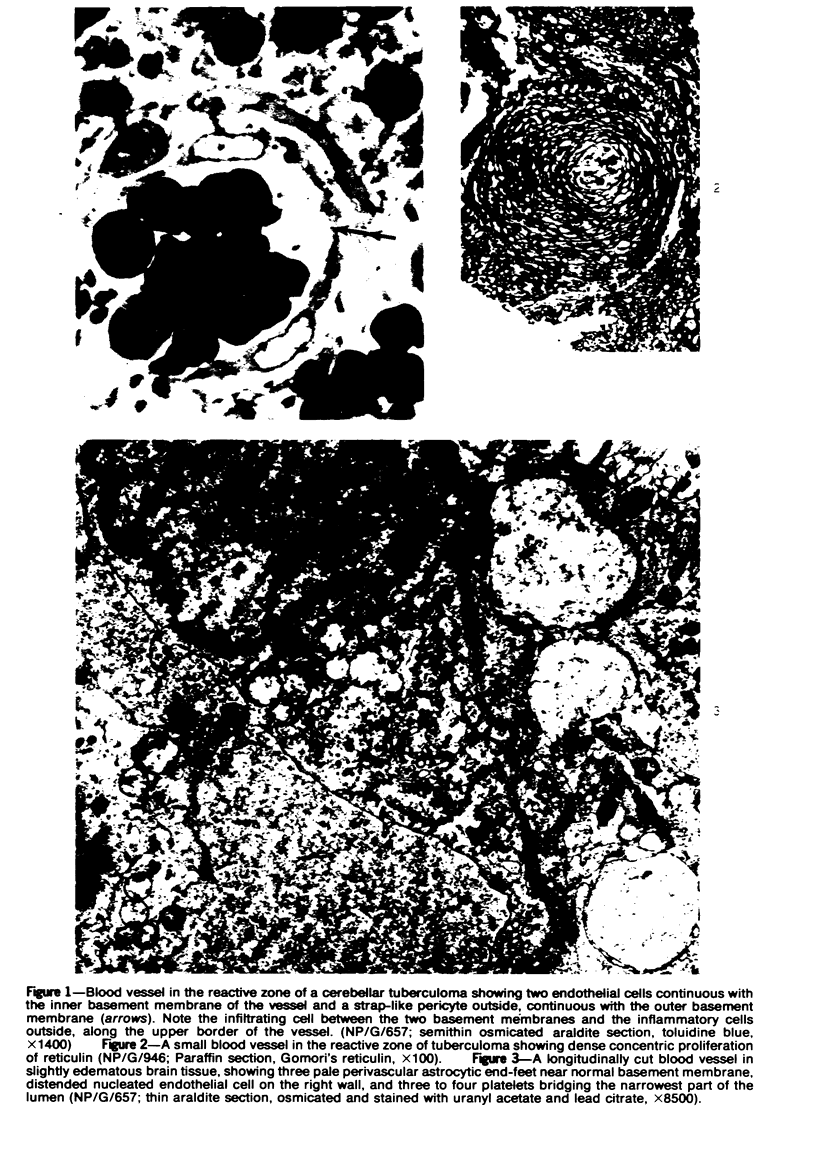
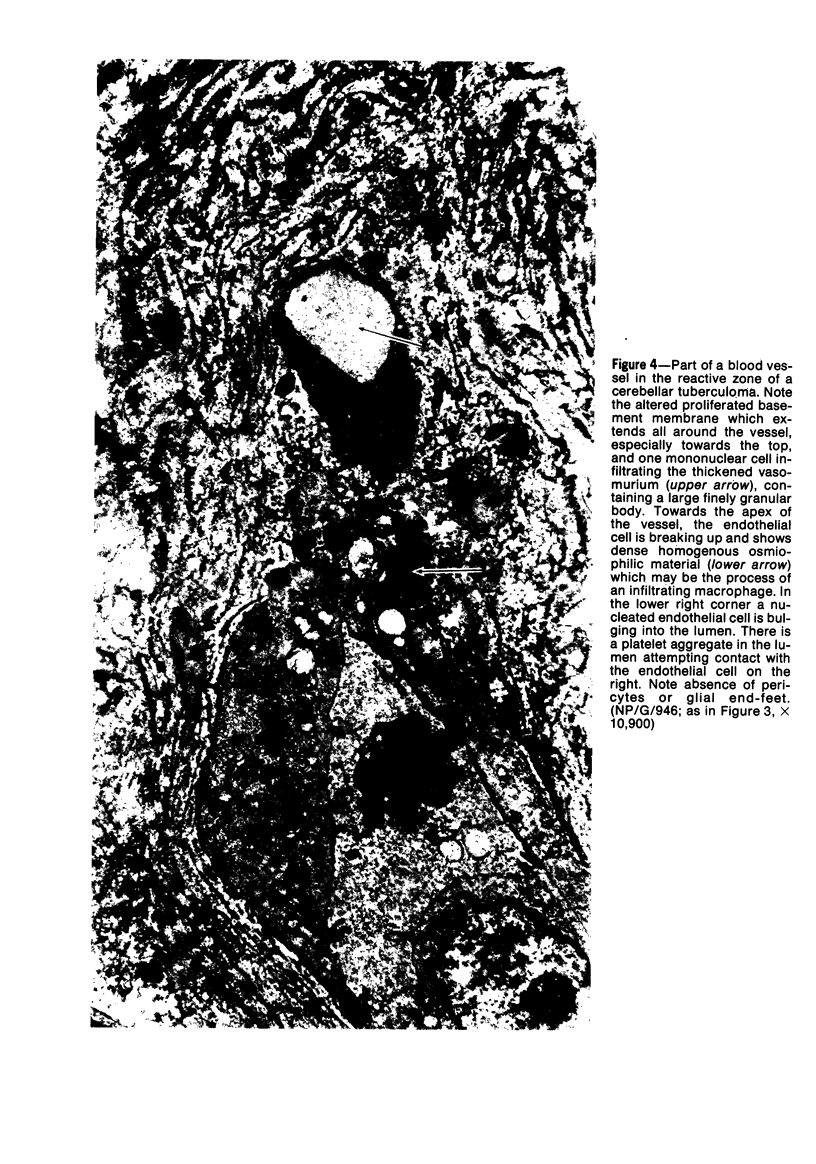
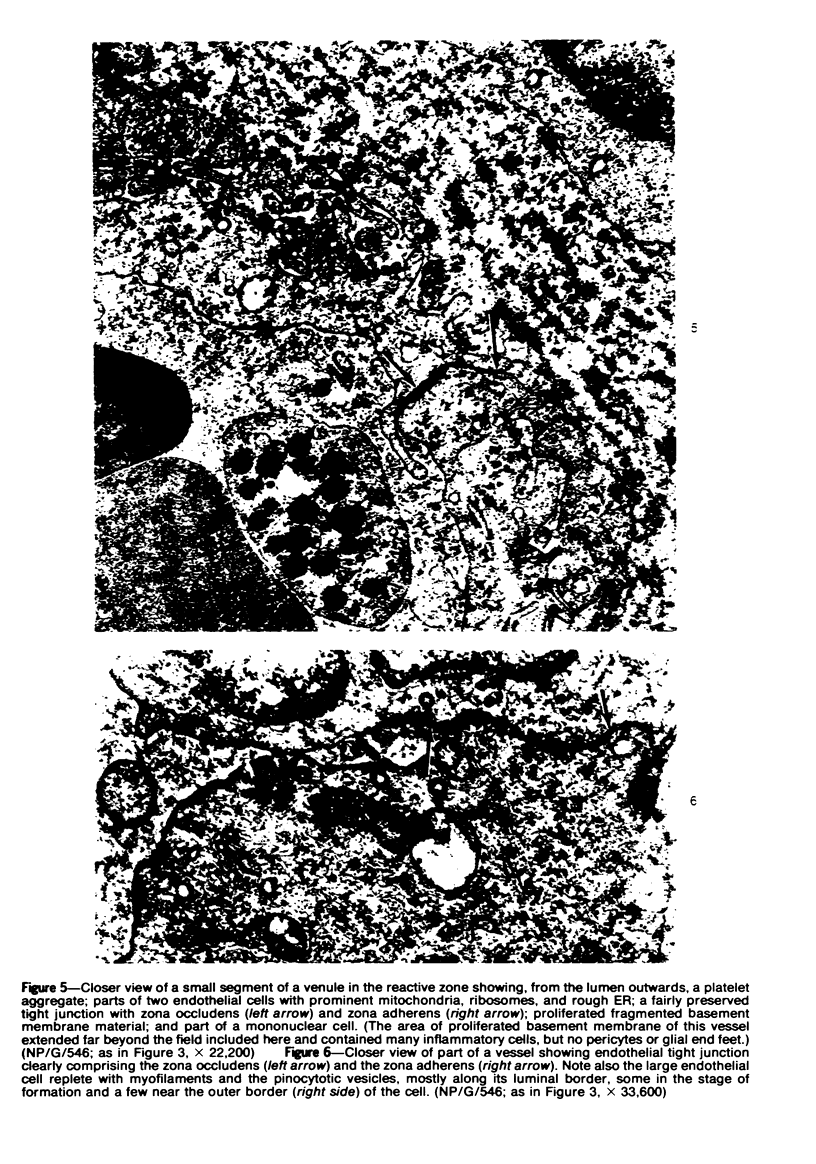

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEU F. P., EDELMAN F. L., KATZMAN R., SCHEINBERG L. C. ULTRASTRUCTURAL AND BIOCHEMICAL ANALYSIS IN CEREBRAL EDEMA ASSOCIATED WITH EXPERIMENTAL MOUSE GLIOMAS. J Neuropathol Exp Neurol. 1964 Apr;23:253–263. doi: 10.1097/00005072-196404000-00002. [DOI] [PubMed] [Google Scholar]
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Ashton N. Vascular basement membrane changes in diabetic retinopathy. Montgomery lecture, 1973. Br J Ophthalmol. 1974 Apr;58(4):344–366. doi: 10.1136/bjo.58.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddingius J. Ultrastructural changes in blood vessels of peripheral nerves in leprosy neuropathy. I. Tuberculoid and borderline-tuberculoid leprosy patients. Acta Neuropathol. 1976 Jun 15;35(2):159–181. doi: 10.1007/BF00690562. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J. Elektronenmikroskopie der Hämangioblastome des ZNS und der angioblastischen Meningiome. Acta Neuropathol. 1971;19(3):184–207. doi: 10.1007/BF00684596. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro, Matakas F. The ulstratructure of reticulin. Acta Neuropathol Suppl. 1975;Suppl 6:173–176. [PubMed] [Google Scholar]
- Dastur D. K., Udani P. M. The pathology and pathogenesis of tuberculous encephalopathy. Acta Neuropathol. 1966 Jul 7;6(4):311–326. doi: 10.1007/BF00688161. [DOI] [PubMed] [Google Scholar]
- Dastur D., Wadia N. H. Spinal meningitides with radiculo-myelopathy. 2. Pathology and pathogenesis. J Neurol Sci. 1969 Mar-Apr;8(2):261–297. doi: 10.1016/0022-510x(69)90113-0. [DOI] [PubMed] [Google Scholar]
- Dixon F. J. The pathogenesis of glomerulonephritis. Am J Med. 1968 Apr;44(4):493–498. doi: 10.1016/0002-9343(68)90050-8. [DOI] [PubMed] [Google Scholar]
- FLOREY H. W., GRANT L. H. Leucocyte migration from small blood vessels stimulated with ultraviolet light: an electron-microscope study. J Pathol Bacteriol. 1961 Jul;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- GONATAS N. K., ZIMMERMAN H. M., LEVINE S. Ultrastructure of inflammation with edema in the rat brain. Am J Pathol. 1963 Apr;42:455–469. [PMC free article] [PubMed] [Google Scholar]
- Garcia J. H., Cox J. V., Hudgins W. R. Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropathol. 1971;18(4):273–285. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- Hills C. P. Ultrastructural changes in the capillary bed of the rat cerebral cortex in anoxic-ischemic brain lesions. Am J Pathol. 1964 Apr;44(4):531–551. [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Tomiyasu U., Zimmerman H. M. The fine structure of blood vessels in chromophobe adenoma. Acta Neuropathol. 1972;22(3):200–207. doi: 10.1007/BF00684523. [DOI] [PubMed] [Google Scholar]
- Long D. M. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970 Feb;32(2):127–144. doi: 10.3171/jns.1970.32.2.0127. [DOI] [PubMed] [Google Scholar]
- Long D. M., Hartmann J. F., French L. A. The ultrastructure of human cerebral edema. J Neuropathol Exp Neurol. 1966 Jul;25(3):373–395. doi: 10.1097/00005072-196607000-00003. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE TERMINAL VASCULAR BED. IV. THE VENULES AND THEIR PERIVASCULAR CELLS (PERICYTES, ADVENTITIAL CELLS). Exp Mol Pathol. 1964 Apr;34:98–114. doi: 10.1016/0014-4800(64)90044-9. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., Jr, MIDGLEY A. R., Jr, SRI RAM J. The histogenesis of basement membranes. J Exp Med. 1963 Mar 1;117:339–348. doi: 10.1084/jem.117.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M., Miquel J., Weibel J. The short- and long-term effects of ultraviolet irradiation on the exposed cat cerebrum. Light-microscopic, enzyme-histochemical and fine-structural observations. J Neurol Sci. 1971 Jul;13(3):351–375. doi: 10.1016/0022-510x(71)90038-4. [DOI] [PubMed] [Google Scholar]
- Williams V., Grossman R. G. Ultrastructure of cortical synapses after failure of presynaptic activity in ischemia. Anat Rec. 1970 Feb;166(2):131–141. doi: 10.1002/ar.1091660202. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bloom B. R. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med. 1975 Feb 1;141(2):346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]