Abstract
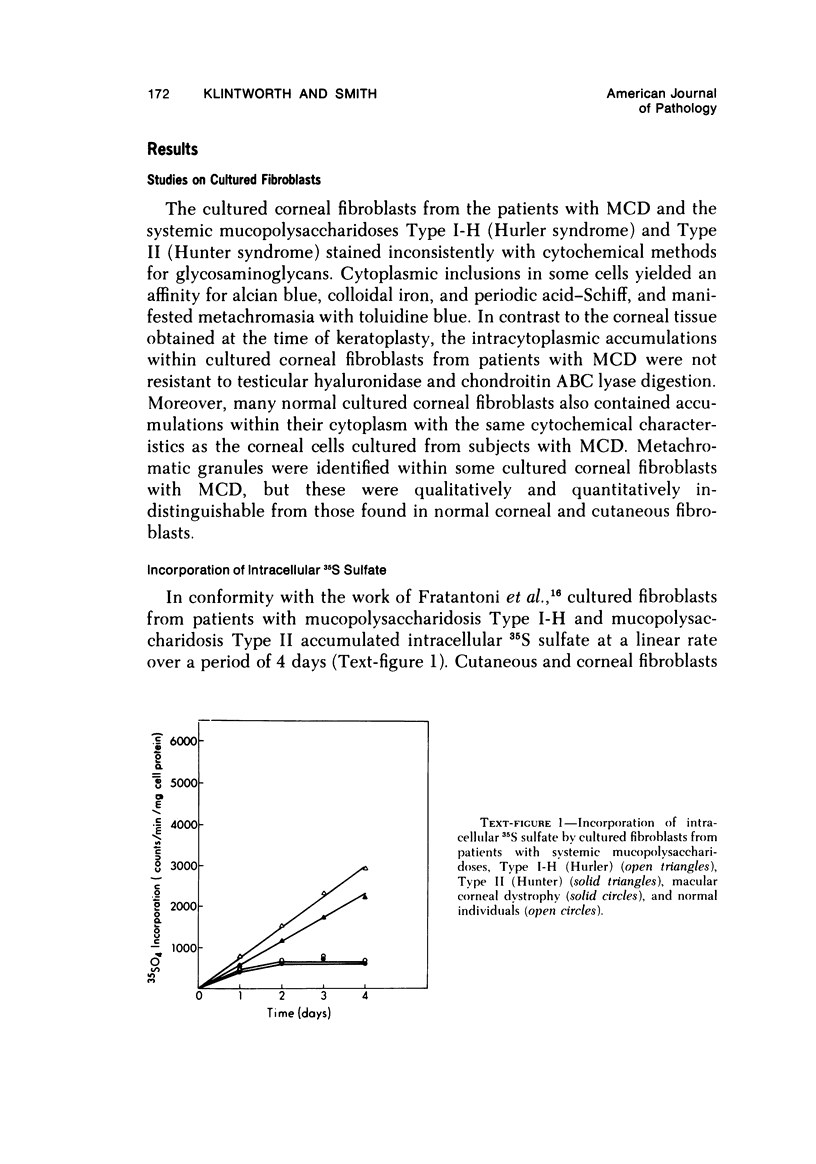
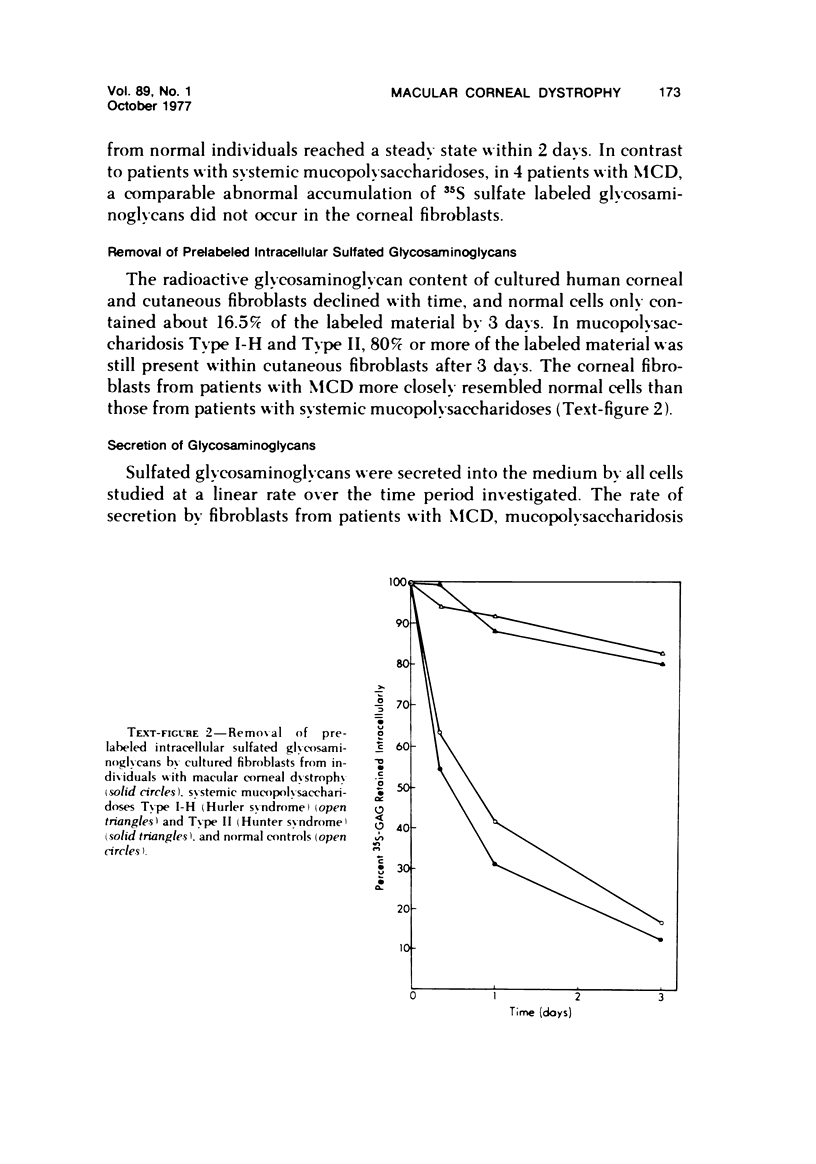
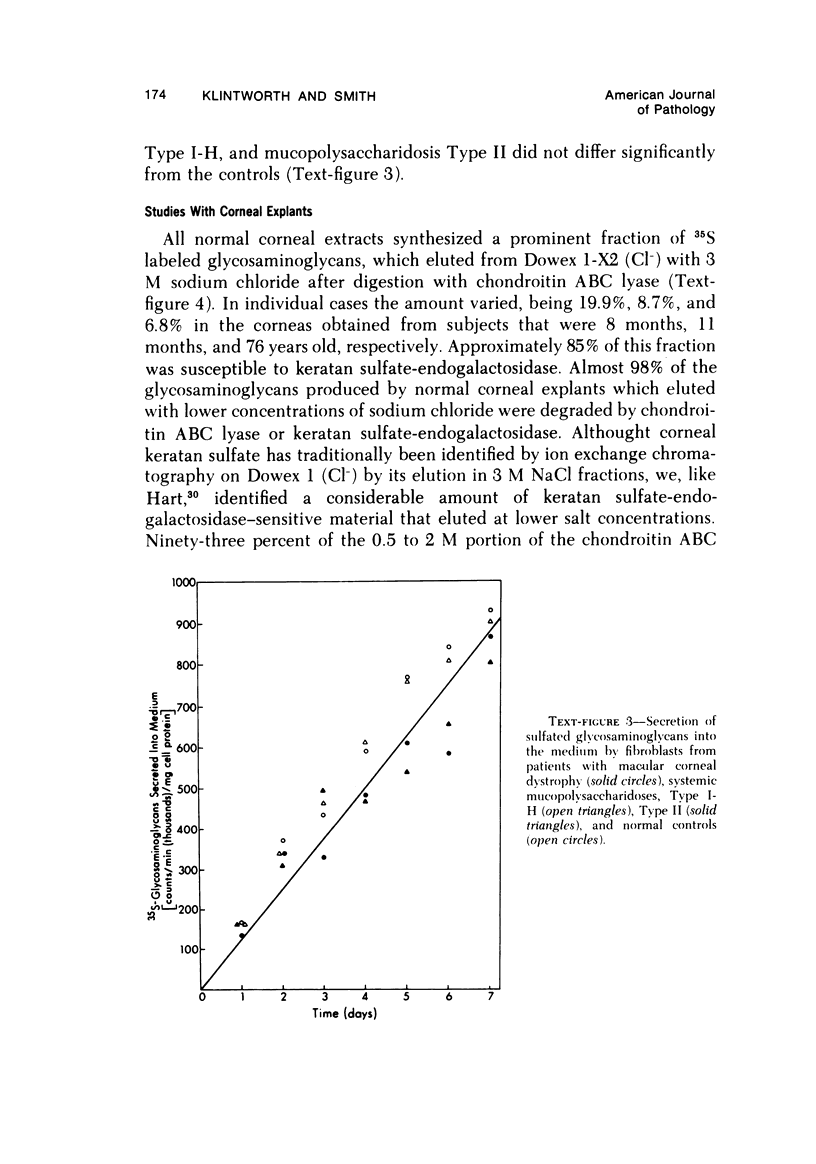
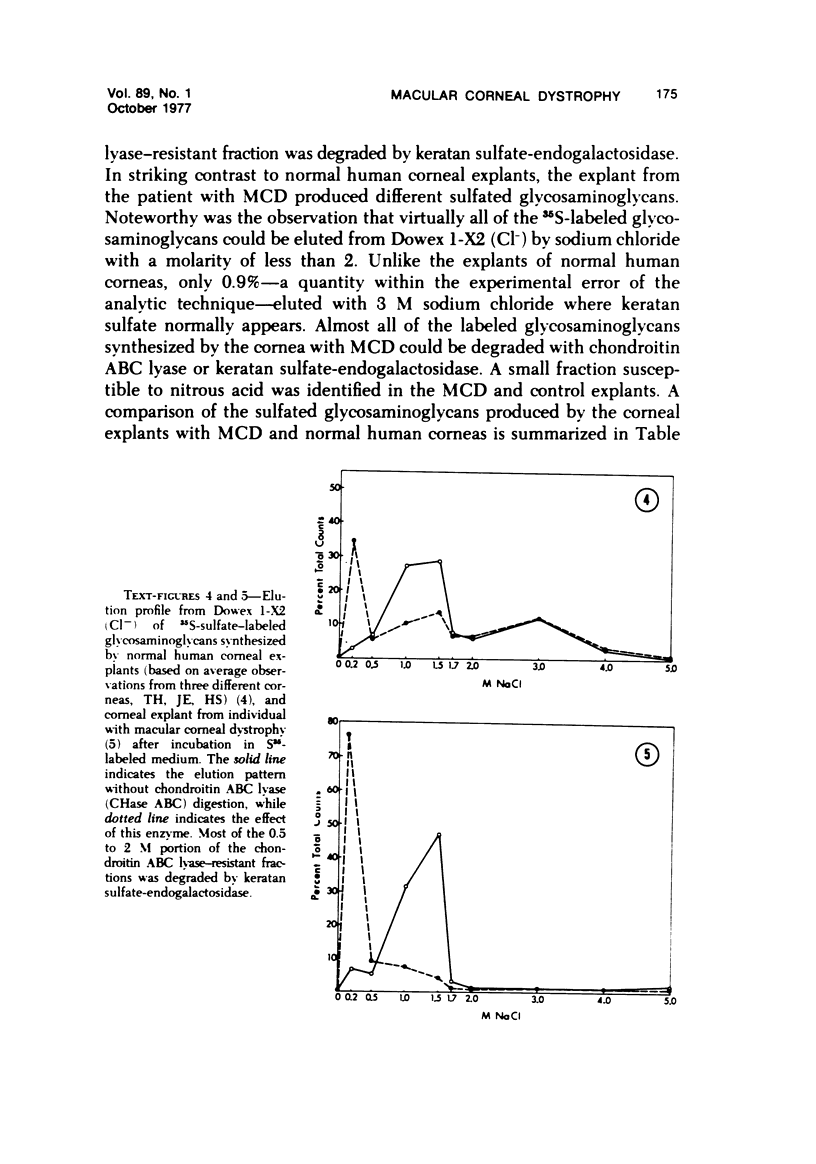
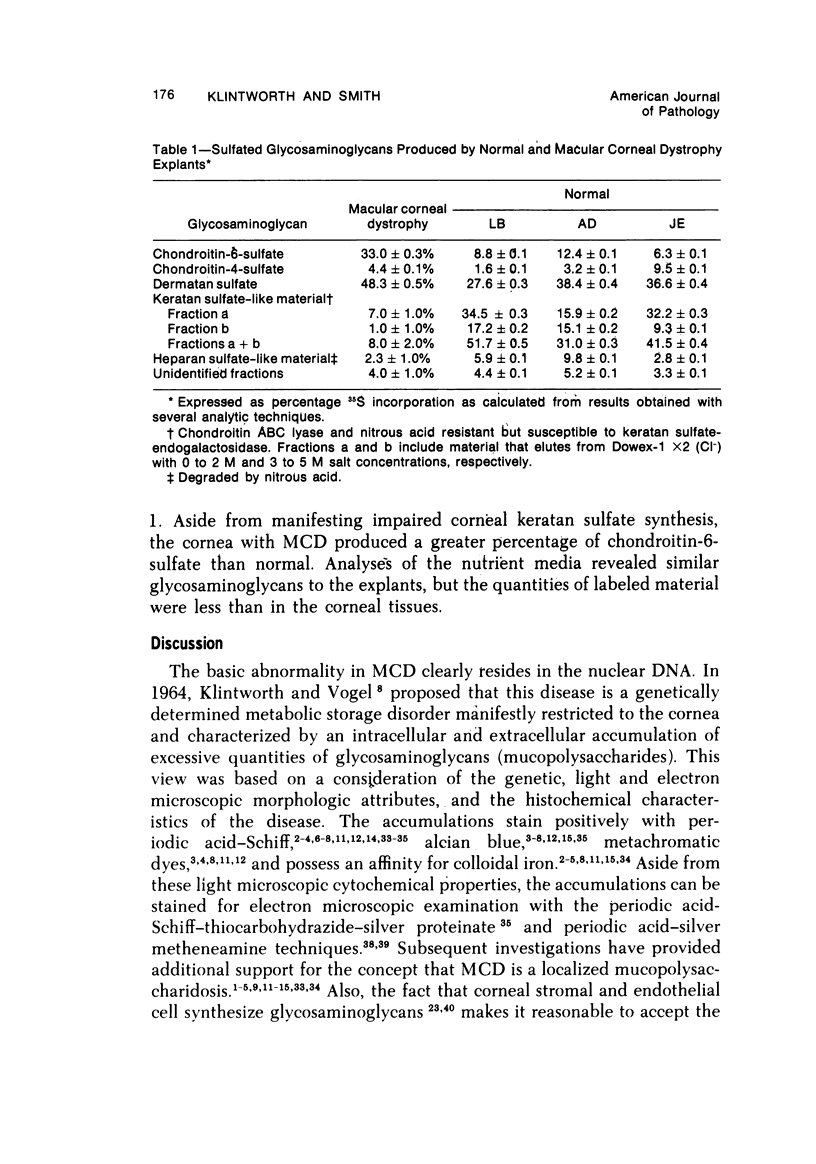
The inherited disorder macular corneal dystrophy (MCD), a localized corneal mucopolysaccharidosis, is currently thought to result from an inability to catabolize corneal keratan sulfate (keratan sulfate 1). As studies on isolated cells have provided insight into metabolic abnormalities in other inherited disorders, we investigated cultured corneal fibroblasts from 4 patients with MCD from several standpoints. Lines of corneal fibroblasts with MCD could not be distinguished from controls with cytochemical methods known to stain the naturally occurring accumulations. In contrast to cultured fibroblasts from patients with mucopolysaccharidoses Type I-H (Hurler syndrome) and Type II (Hunter syndrome), corneal fibroblasts from patients with MCD did not accumulate abnormal quantities of 35S-sulfate-labeled glycosaminoglycans, but like normal corneal and cutaneous fibroblasts reached a state of equilibrium within 2 days. Also, the rate at which sulfated glycosaminoglycans were removed from cultured corneal fibroblasts in MCD by secretion and degradation more closely resembled that of normal cells than those with the systemic mucopolysaccharidoses. The secretion of sulfated glycosaminoglycans into the nutrient medium by corneal fibroblasts from patients with MCD occurred at a linear rate comparable to that of other cells studied. The aforementioned data, nonetheless, remain consistent with the hypothesis that MCD is an inherited disorder of keratan sulfate I (corneal keratan sulfate) catabolism, as isolated corneal fibroblasts in contrast to corneal explants synthesize little or no keratan sulfate in culture. In view of the latter, we also compared the profile of 35S-labeled glycosaminoglycans produced by a corneal explant from a patient with MCD with that normally synthesized by human corneal explants. The latter synthesized and secreted a population of 35S-sulfate-labeled glycosaminoglycans with properties of keratan sulfate. Considerably less material with these attributes was identified with the same analytic techniques in the cornea with MCD or in its surrounding medium after the abnormal cornea had been incubated under identical conditions. In addition to manifesting an impaired synthesis of corneal keratan sulfate-like material, the cornea with MCD produced a greater percentage of chondroitin-6-sulfate than normal. These findings suggest that the synthesis of corneal keratan sulfate and other glycosaminoglycans may be altered in MCD.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhavanandan V. P., Meyer K. Studies on keratosulfates. Methylation and partial acid hydrolysis of bovine corneal keratosulfate. J Biol Chem. 1967 Oct 10;242(19):4352–4359. [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke S., Thiel H. J., Niedorf H. R. Licht- und elektronenmikroskopische Untersuchungen über die fleckförmige Hornhautdystrophie. Ophthalmologica. 1972;164(1):35–49. doi: 10.1159/000306702. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. Structural studies on heparins with unusually high N-acetylglucosamine contents. Biochim Biophys Acta. 1973 Sep 14;320(2):331–340. doi: 10.1016/0304-4165(73)90313-9. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Dorfman A. Synthesis of sulfated mucopolysaccharides by chick corneal fibroblasts in vitro. Exp Eye Res. 1974 May;18(5):421–433. doi: 10.1016/0014-4835(74)90079-7. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Hart G. W. Heparan sulfate biosynthesis by embryonic tissues and primary fibroblast populations. Dev Biol. 1975 Jun;44(2):253–269. doi: 10.1016/0012-1606(75)90396-6. [DOI] [PubMed] [Google Scholar]
- Doetsch K., Gadsden R. H. Determination of urinary total protein by use of gel filtration and a modified biuret method. Clin Chem. 1975 May;21(6):778–781. [PubMed] [Google Scholar]
- François J., Victoria-Troncoso V., Maudgal P. C., Victoria-Ihler A. Study of the lysosomes by vital stains in normal keratocytes and in keratocytes from macular dystrophy of the cornea. Invest Ophthalmol. 1976 Aug;15(8):599–605. [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A. Histochemistry of corneal macular dystrophy. Invest Ophthalmol. 1969 Oct;8(5):475–483. [PubMed] [Google Scholar]
- Ghosh M., McCulloch C. Macular corneal dystrophy. Can J Ophthalmol. 1973 Oct;8(4):515–526. [PubMed] [Google Scholar]
- Graf B., Pouliquen Y., Frouin M. A., Faure J. P., Offret G. Cytochemical study of macular dystrophy of the cornea (Groenouw II): an ultrastructural study. Exp Eye Res. 1974 Feb;18(2):163–169. doi: 10.1016/0014-4835(74)90103-1. [DOI] [PubMed] [Google Scholar]
- Hart G. W. Biosynthesis of glycosaminolgycans during corneal development. J Biol Chem. 1976 Nov 10;251(21):6513–6521. [PubMed] [Google Scholar]
- Herrmann J., Meythaler H. Licht- und elektronenmikroskopische Untersuchungen bei Dystrophia corneae maculosa. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1971;181(2):165–178. doi: 10.1007/BF00414757. [DOI] [PubMed] [Google Scholar]
- Hirano S., Meyer K. Enzymatic degradation of corneal and cartilaginous keratosulfates. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1371–1375. doi: 10.1016/s0006-291x(71)80237-1. [DOI] [PubMed] [Google Scholar]
- JENNINGS M. A., FLOREY H. W. Autoradiographic observations on the mucous cells of the stomach and intestine. Q J Exp Physiol Cogn Med Sci. 1956 Apr;41(2):131–152. doi: 10.1113/expphysiol.1956.sp001171. [DOI] [PubMed] [Google Scholar]
- JONES S. T., ZIMMERMAN L. E. Histopathologic differentiation of granular, macular and lattice dystrophies of the cornea. Am J Ophthalmol. 1961 Mar;51:394–410. doi: 10.1016/0002-9394(61)92085-2. [DOI] [PubMed] [Google Scholar]
- JONES S. T., ZIMMERMAN L. E. Macular dystrophy of the cornea (Groenouw type II); clinicopathologic report of two cases with comments concerning its differential diagnosis from lattice dystrophy (Biber-Haab-Dimmer). Am J Ophthalmol. 1959 Jan;47(1 Pt 1):1–16. [PubMed] [Google Scholar]
- KLINTWORTH G. K., VOGEL F. S. MACULAR CORNEAL DYSTROPHY. AN INHERITED ACID MUCOPOLYSACCHARIDE STORAGE DISEASE OF THE CORNEAL FIBROBLAST. Am J Pathol. 1964 Oct;45:565–586. [PMC free article] [PubMed] [Google Scholar]
- Klintworth G. K. Current concepts on the ultrastructural pathogenesis of macular and lattice corneal dystrophies. Birth Defects Orig Artic Ser. 1971 Mar;7(3):27–31. [PubMed] [Google Scholar]
- Klintworth G. K., Smith C. F. A comparative study of extracellular sulfated glycosaminoglycans synthesized by rabbit corneal fibroblasts in organ and confluent cultures. Lab Invest. 1976 Sep;35(3):258–263. [PubMed] [Google Scholar]
- Klintworth G. K. Tissue culture in the inherited corneal dystrophies: possible applications and problems. Birth Defects Orig Artic Ser. 1976;12(3):115–132. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lie S. O., McKusick V. A., Neufeld E. F. Simulation of genetic mucopolysaccharidoses in normal human fibroblasts by alteration of pH of the medium. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2361–2363. doi: 10.1073/pnas.69.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti D. W., Kaufman H. E. Macular and lattice dystrophies and their recurrences after keratoplasty. Trans Am Acad Ophthalmol Otolaryngol. 1967 Jan-Feb;71(1):112–118. [PubMed] [Google Scholar]
- Mathews M. B., Cifonelli J. A. Comparative biochemistry of keratosulfates. J Biol Chem. 1965 Nov;240(11):4140–4145. [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Morgan G. Macular dystrophy of the cornea. Br J Ophthalmol. 1966 Feb;50(2):57–67. doi: 10.1136/bjo.50.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Okada T. S., Eguchi G., Takeichi M. The expression of differentiation by chicken lens epithelium in in vitro cell culture. Dev Growth Differ. 1971 Dec;13(4):323–336. doi: 10.1111/j.1440-169x.1971.00323.x. [DOI] [PubMed] [Google Scholar]
- PASTERNAK C. A., KENT P. W. Biosynthesis of intestinal mucins. 2. Incorporation of [35S] sulphate by guinea-pig colon in vitro. Biochem J. 1958 Mar;68(3):452–457. doi: 10.1042/bj0680452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Oda M., Edwards V. D., Greenberg G. R., Jeejeebhoy K. N. Ultrastructural and functional studies of cultured hepatocytes. Lab Invest. 1974 Nov;31(5):533–542. [PubMed] [Google Scholar]
- Rodesch F. Differentiation, contact inhibition and intercellular communication in retinal pigment cells. Exp Cell Res. 1973 Jan;76(1):55–62. doi: 10.1016/0014-4827(73)90418-7. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Seitz R., Goslar H. G. Beitrag zur Klinik, Morphologie und Histochemie der verschiedenen Formen von Hornhautdystrophie. Klin Monbl Augenheilkd. 1965 Dec;147(5):673–691. [PubMed] [Google Scholar]
- Seno N., Anno K., Kondo K., Nagase S., Saito S. Improved method for electrophoretic separation and rapid quantitation of isomeric chondroitin sulfates on cellulose acetate strips. Anal Biochem. 1970 Sep;37(1):197–202. doi: 10.1016/0003-2697(70)90280-0. [DOI] [PubMed] [Google Scholar]
- Snip R. C., Kenyon K. R., Green W. R. Macular corneal dystrophy: ultrastructural pathology of corneal endothelium and Descemet's membrane. Invest Ophthalmol. 1973 Feb;12(2):88–97. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins: their biochemistry, biology and role in human disease (first of two parts). N Engl J Med. 1969 Oct 30;281(18):991–contd. doi: 10.1056/NEJM196910302811806. [DOI] [PubMed] [Google Scholar]
- Teng C. C. Macular dystrophy of the cornea. A histochemical and electron microscopic study. Am J Ophthalmol. 1966 Sep;62(3):436–454. doi: 10.1016/0002-9394(66)91323-7. [DOI] [PubMed] [Google Scholar]
- Tremblay M., Dubé I. Macular dystrophy of the cornea. Ultrastructure of two cases. Can J Ophthalmol. 1973 Jan;8(1):47–53. [PubMed] [Google Scholar]
- Tripathi R. C., Ashton N. Application of electron microscopy to the study of ocular inborn errors of metabolism. Birth Defects Orig Artic Ser. 1976;12(3):69–104. [PubMed] [Google Scholar]
- Yue B. Y., Baum J. L. The synthesis of glycosaminoglycans by cultures of rabbit corneal endothelial and stromal cells. Biochem J. 1976 Sep 15;158(3):567–573. doi: 10.1042/bj1580567. [DOI] [PMC free article] [PubMed] [Google Scholar]


