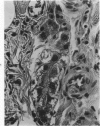
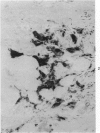
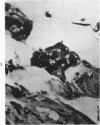
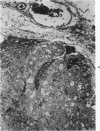
Abstract
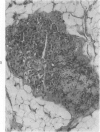
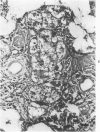
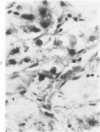
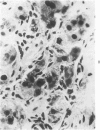
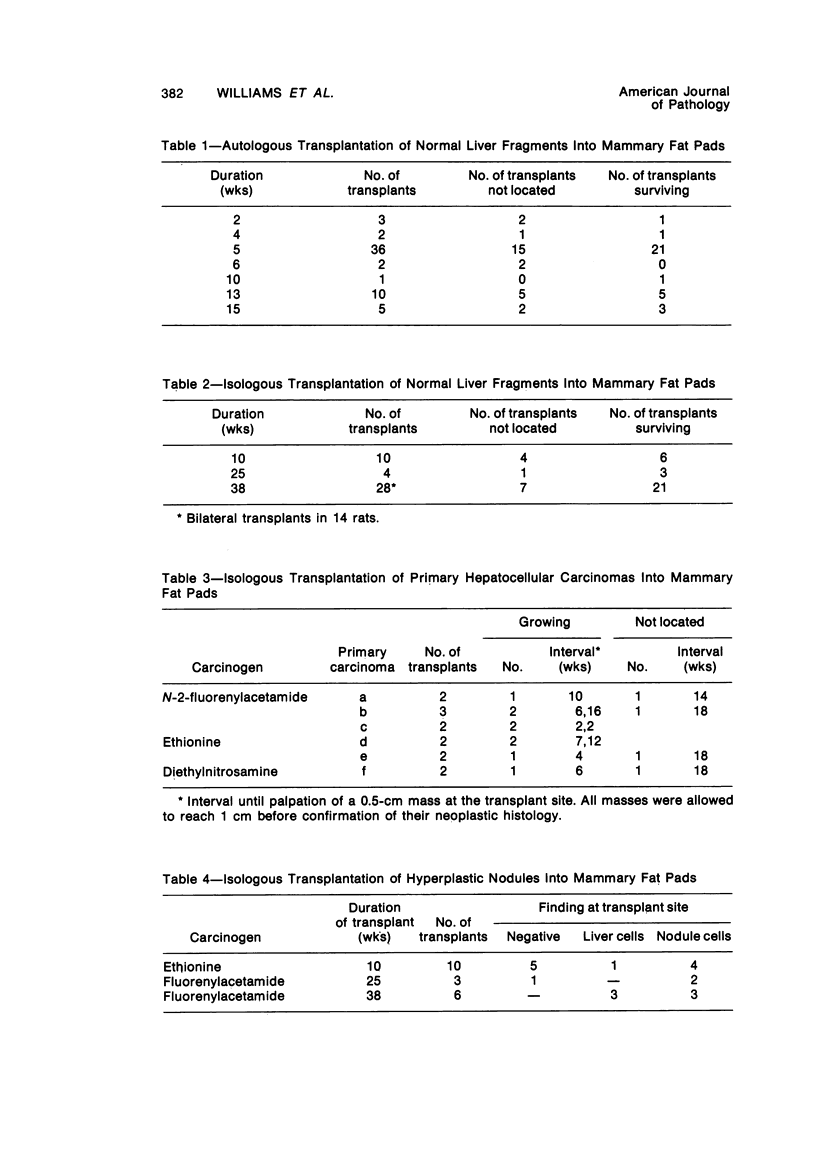
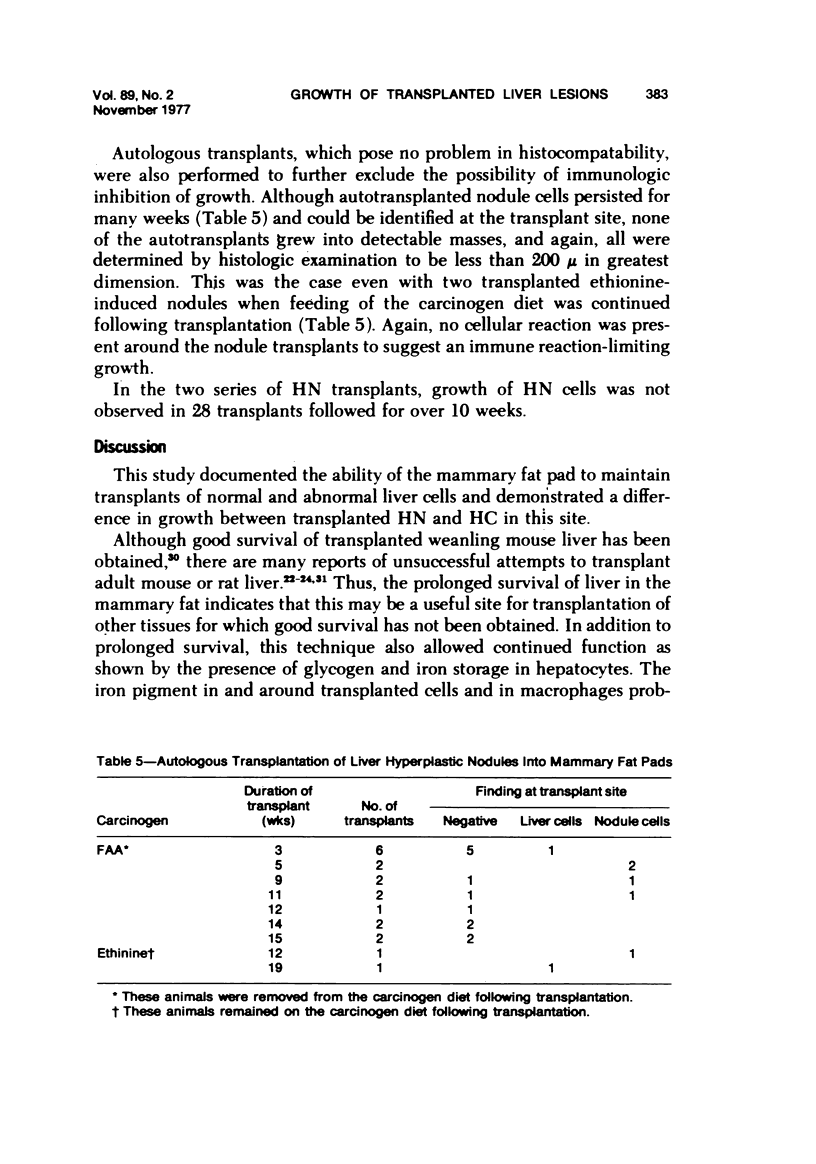
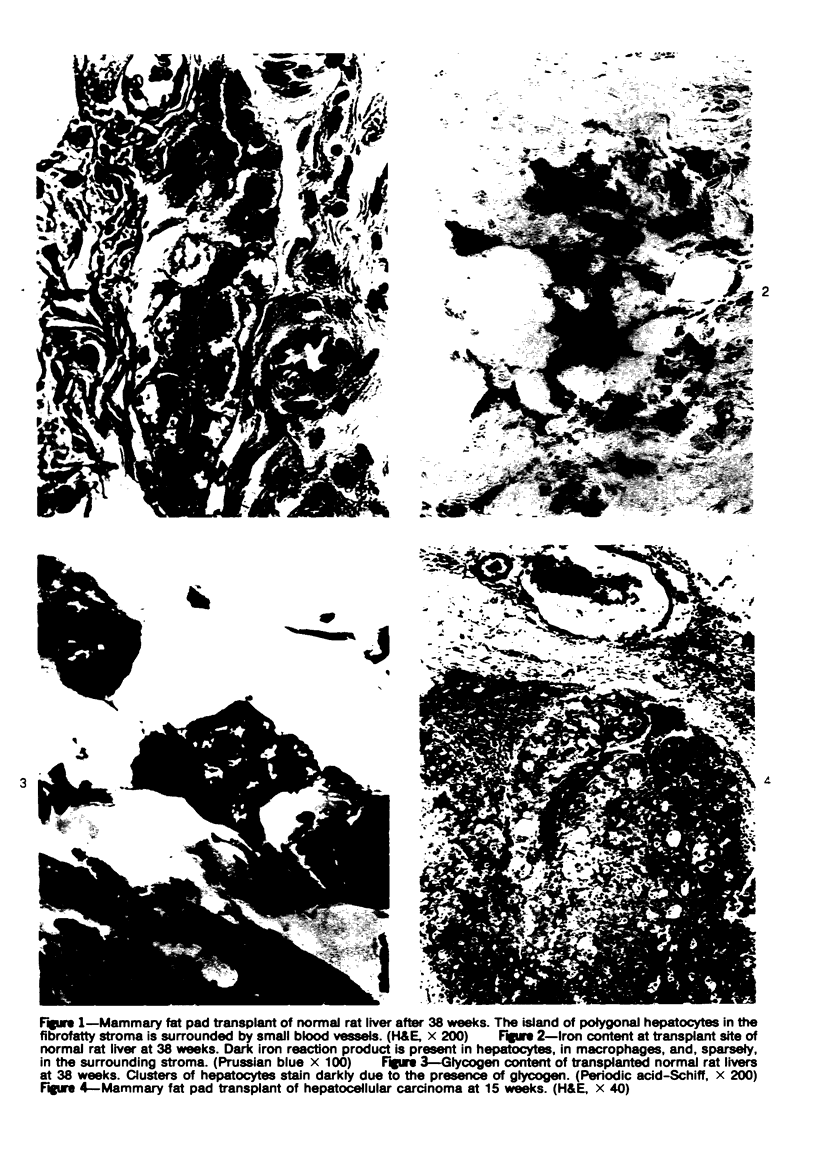
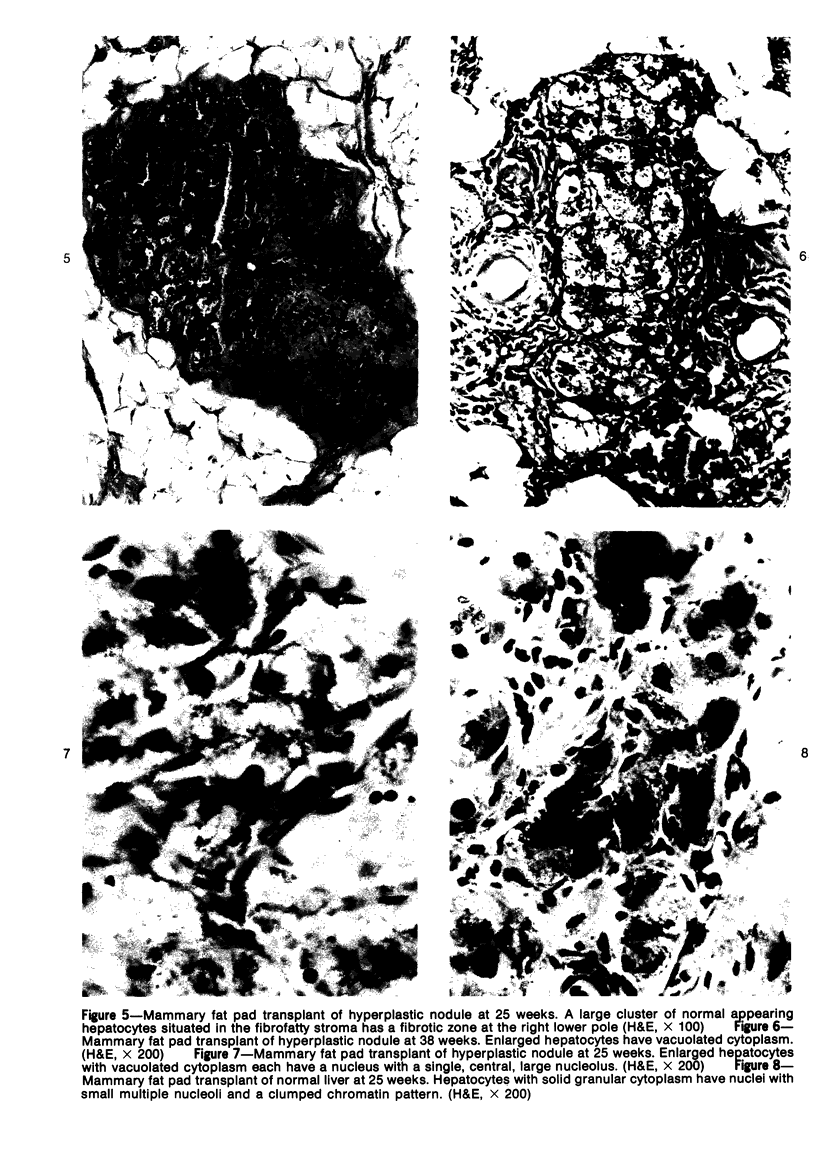
Transplantation of fragments of normal rat liver autologously and isologously into the inguinal mammary fat pad permitted survival for up to 75% of grafts for 38 weeks, the longest interval studied. Similarly transplanted hepatocarcinomas grew rapidly and progressively in this site. Neither autologous or isologous transplants of liver hyperplastic nodules displayed obvious growth, although like normal liver, they also persisted for up to 38 weeks. Some persisting hyperplastic cells retained certain characteristic features, but others appeared to revert to a normal morphology. Thus, there is a stage in which hyperplastic cells do not possess the progressive growth ability of neoplastic cells and appear to be capable of reversion to a normal phenotype.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berneman A., Ellouz R., Lenfant M., Giralt E. F. Inhibition de l'incorporation de thymidine trité dans le foie foetal de rat en culture cellulaire par un extrait hépatique fractionné. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jul 21;281(2-3):187–190. [PubMed] [Google Scholar]
- Calvert J. C., Liebelt A. G., Liebelt R. A. Influence of anatomic site, donor age and genetics on liver implantation in the mouse. Tex Rep Biol Med. 1972 Winter;30(4):287–299. [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- DIPAOLO J. A. CHROMOSOME PATTERNS IN MOUSE LUNG TUMORS. J Natl Cancer Inst. 1965 Mar;34:337–343. [PubMed] [Google Scholar]
- Epstein S., Ito N., Merkow L., Farber E. Cellular analysis of liver carcinogenesis: the induction of large hyperplastic nodules in the liver with 2-fluorenylacetamide or ethionine and some aspects of their morphology and glycogen metabolism. Cancer Res. 1967 Sep;27(9):1702–1711. [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- FARBER E., ICHINOSE H. On the origin of malignant cells in experimental liver cancer. Acta Unio Int Contra Cancrum. 1959;15(1):152–153. [PubMed] [Google Scholar]
- FIRMINGER H. I. Histopathology of carcinogenesis and tumors of the liver in rats. J Natl Cancer Inst. 1955 Apr;15(5 Suppl):1427–1442. [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- GOLDFARB S., ZAK F. G. Role of injury and hyperplasia in the induction of hepatocellular carcinoma. JAMA. 1961 Nov 18;178:729–731. doi: 10.1001/jama.1961.73040460007007b. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., LEONG G. F., HOLE B. V. HETEROTOPIC PARTIAL AUTOTRANSPLANTATION OF RAT LIVER: TECHNIC AND DEMONSTRATION OF STRUCTURE AND FUNCTION OF THE GRAFT. Cancer Res. 1964 Sep;24:1474–1495. [PubMed] [Google Scholar]
- Kimura K. Progression of pulmonary tumor in mice. 1. Histological studies of primary and transplanted pulmonary tumors. Acta Pathol Jpn. 1971 Feb;21(1):13–56. doi: 10.1111/j.1440-1827.1971.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Pitot H. C. The regulation of serine dehydratase and glucose-6-phosphatase in hyperplastic nodules of rat liver during diethylnitrosamine and N-2-fluorenylacetamide feeding. Cancer Res. 1975 Apr;35(4):1075–1084. [PubMed] [Google Scholar]
- Kitagawa T. Responsiveness of hyperplastic lesions and hepatomas to partial hepatectomy. Gan. 1971 Jun;62(3):217–224. [PubMed] [Google Scholar]
- LAWS J. O. Tissue regeneration and tumour development. Br J Cancer. 1959 Dec;13:669–674. doi: 10.1038/bjc.1959.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDUC E. H., WILSON J. W. Production of transplantable hepatomas by intrasplenic implantation of normal liver in the mouse. J Natl Cancer Inst. 1963 Jan;30:85–99. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Nadal C., Lombard M. N., Zajdela F. Inhibition of rat hepatocyte multiplication by serum and liver factors: physiological development and experimental induction. Virchows Arch B Cell Pathol. 1976 May 26;20(4):277–285. doi: 10.1007/BF02890346. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Wogan G. N. Sequential morphologic changes in aflatoxin B carcinogenesis in the rat. Cancer Res. 1968 Apr;28(4):770–781. [PubMed] [Google Scholar]
- POPPER H., STERNBERG S. S., OSER B. L., OSER M. The carcinogenic effect of aramite in rats. A study of hepatic nodules. Cancer. 1960 Sep-Oct;13:1035–1046. doi: 10.1002/1097-0142(196009/10)13:5<1035::aid-cncr2820130526>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- REUBER M. D., FIRMINGER H. I. MORPHOLOGIC AND BIOLOGIC CORRELATION OF LESIONS OBTAINED IN HEPATIC CARCINOGENESIS IN A X C RATS GIVEN 0.025 PERCENT N-2-FLUORENYLDIACETAMIDE. J Natl Cancer Inst. 1963 Dec;31:1407–1429. [PubMed] [Google Scholar]
- Rabes H., Hartenstein R., Scholze P. Specific stages of cellular response to homeostatic control during diethylnitrosamine-induced liver carcinogenesis. Experientia. 1970 Dec 15;26(12):1356–1359. doi: 10.1007/BF02113030. [DOI] [PubMed] [Google Scholar]
- Reuber M. D. Development of preneoplastic and neoplastic lesions of the liver in male rats given 0.025 percent N-2-fluorenyldiacetamide. J Natl Cancer Inst. 1965 Jun;34(6):697–723. [PubMed] [Google Scholar]
- Reuber M. D., Odashima S. Further studies on the transplantation of lesions in hepatic carcinogenesis in rats given 2-(diacetamido)fluorene. Gan. 1967 Dec;58(6):513–520. [PubMed] [Google Scholar]
- Squire R. A., Levitt M. H. Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Res. 1975 Nov;35(11 Pt 1):3214–3223. [PubMed] [Google Scholar]
- Tavassoli M., Crosby W. H. The fate of fragments of liver implanted in ectopic sites. Anat Rec. 1970 Feb;166(2):143–151. doi: 10.1002/ar.1091660203. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Verly W. G., Deschamps Y., Pushpathadam J., Desrosiers M. The hepatic chalone. I. Assay method for the hormone and purification of the rabbit liver chalone. Can J Biochem. 1971 Dec;49(12):1376–1383. doi: 10.1139/o71-198. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Elliott J. M., Weisburger J. H. Carcinoma after malignant conversion in vitro of epithelial-like cells from rat liver following exposure to chemical carcinogens. Cancer Res. 1973 Mar;33(3):606–612. [PubMed] [Google Scholar]
- Williams G. M., Klaiber M., Parker S. E., Farber E. Nature of early appearing, carcinogen-induced liver lesions to iron accumulation. J Natl Cancer Inst. 1976 Jul;57(1):157–165. doi: 10.1093/jnci/57.1.157. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Yamamoto R. S. Absence of stainable iron from preneoplastic and neoplastic lesions in rat liver with 8-hydroxyquinoline-induced siderosis. J Natl Cancer Inst. 1972 Sep;49(3):685–692. [PubMed] [Google Scholar]
- Winnail D. S., Williams W. L. Formation of ceroid pigment in subcutaneous implants in mice. Anat Rec. 1968 Apr;160(4):759–772. doi: 10.1002/ar.1091600411. [DOI] [PubMed] [Google Scholar]