Abstract
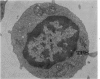
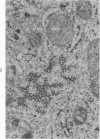
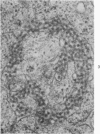
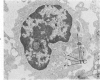
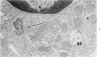
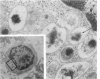
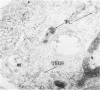
Peripheral blood lymphoid cells obtained from nine twin pairs (six monozygotic and three dizygotic) in which one or both twins had systemic lupus erythematosus (SLE) were examined by electron microscopy for the occurrence of two distinctive intracytoplasmic structures-tubuloreticular structures (TRS) and tubular crystalloids (TC). TRS were found in 0.8 to 14.8% of lymphoid-cell cross sections in 9 of 11 twins with SLE and 2 clinically well but serologically abnormal twins. Lymphoid cells of twins both clinically and serologically normal did not exhibit TRS, although their monozygotic or dizygotic SLE-positive counterparts possessed these structures. Thus, the expression of TRS was more consistent with an acquired than inborn trait and appeared to correlate with disease and serologic manifestations of SLE. TC were found in 1.7 to 7.9% of lymphoid-cell cross sections in every twin examined. No correlation was recognized between clinical or laboratory data and the frequency of TC-bearing cells. The significance and the ultrastructural development of TC in the peripheral blood lymphoid cells are briefly discussed.
Full text
PDF








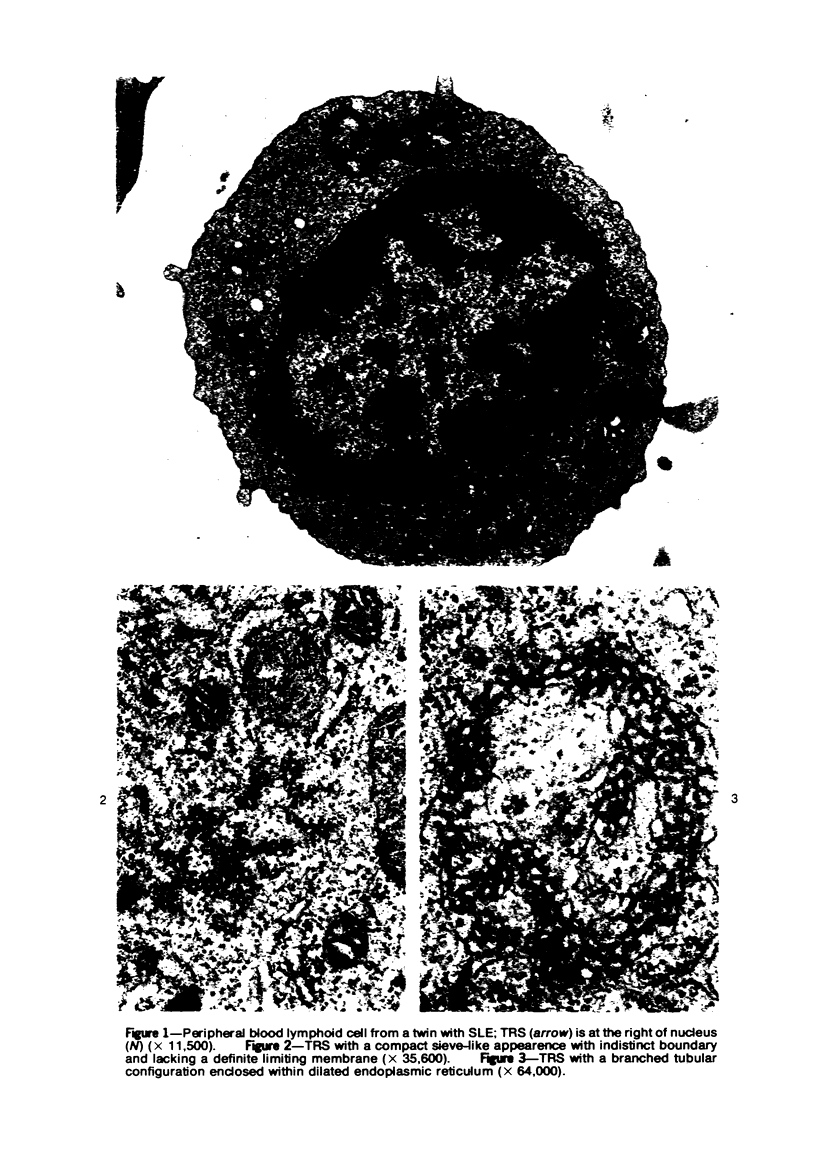
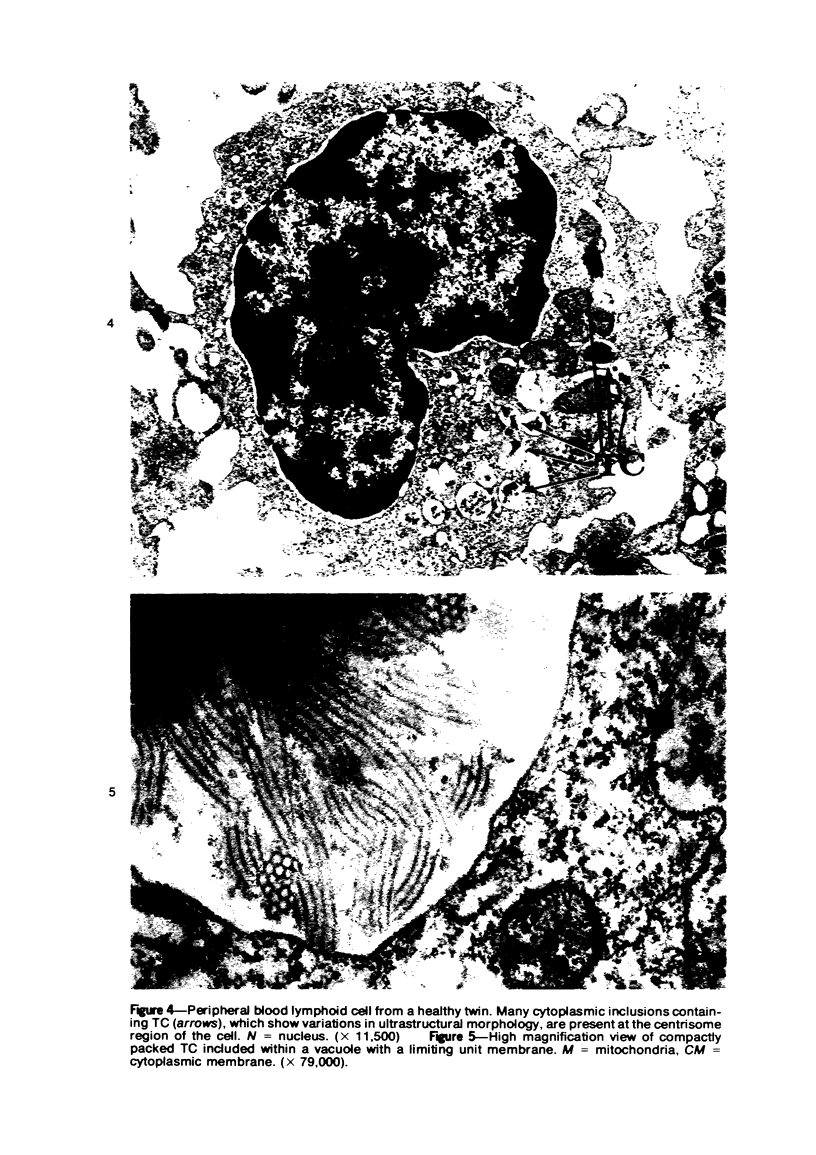
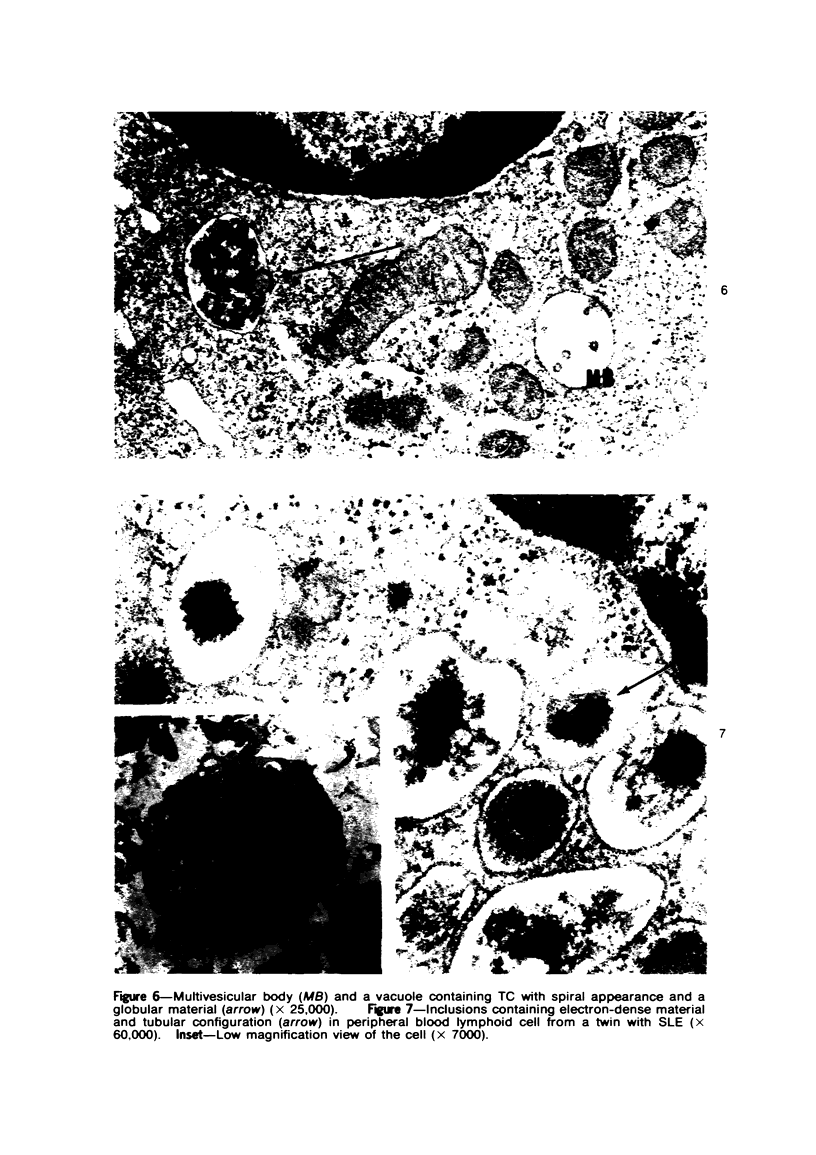

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bariety J., Amor B., Kahan A., Balafrej J. L., Delbarre F. Ultrastructural anomalies in mononuclear cells of peripheral blood in S.L.E.: Presence of virus-like inclusions. Rev Eur Etud Clin Biol. 1971 Aug-Sep;16(7):715–720. [PubMed] [Google Scholar]
- Baringer J. R. Tubular aggregates in endoplasmic reticulum in herpes-simplex encephalitis. N Engl J Med. 1971 Oct 21;285(17):943–945. doi: 10.1056/NEJM197110212851704. [DOI] [PubMed] [Google Scholar]
- Bedoya V., Rabson A. S., Grimley P. M. Growth in vitro of herpes simplex virus in human lymphoma cell lines. J Natl Cancer Inst. 1968 Sep;41(3):635–652. [PubMed] [Google Scholar]
- Belcher R. W., Czarnetzki B. M., Campbell P. B. Ultrastructure of inclusions in peripheral blood mononuclear cells in sarcoidosis. Am J Pathol. 1975 Mar;78(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- Block S. R., Winfield J. B., Lockshin M. D., D'Angelo W. A., Christian C. L. Studies of twins with systemic lupus erythematosus. A review of the literature and presentation of 12 additional sets. Am J Med. 1975 Oct;59(4):533–552. doi: 10.1016/0002-9343(75)90261-2. [DOI] [PubMed] [Google Scholar]
- Christian C. L., Phillips P. E. Viruses and autoimmunity. Am J Med. 1973 May;54(5):611–620. doi: 10.1016/0002-9343(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Daniels T. E., Sylvester R. A., Silverman S., Jr, Polando V., Talal N. Tubuloreticular structures within labial salivary glands in Sjögren's syndrome. Arthritis Rheum. 1974 Sep-Oct;17(5):593–597. doi: 10.1002/art.1780170514. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Hierholzer J. C., Norton W. L. Viral isolation studies of inclusion positive biopsy from human connective tissue diseases. Arthritis Rheum. 1970 Jul-Aug;13(4):378–380. doi: 10.1002/art.1780130403. [DOI] [PubMed] [Google Scholar]
- Givan K. F., Jézéquel A. M. Infectious canine hepatitis: a virologic and ultrastructural study. Lab Invest. 1969 Jan;20(1):36–45. [PubMed] [Google Scholar]
- Gouranton J. Development of an intranuclear nonoccluded rod-shaped virus in some midgut cells of an adult insect, Gyrinus natator L. (Coleoptera). J Ultrastruct Res. 1972 May;39(3):281–294. doi: 10.1016/s0022-5320(72)90023-8. [DOI] [PubMed] [Google Scholar]
- Grrimley P. M., Decker J. L., Michelitch H. J., Frantz M. M. Abnormal structures in circulating lymphocytes from patients with systemic lupus erythematosus and related diseases. Arthritis Rheum. 1973 May-Jun;16(3):313–323. doi: 10.1002/art.1780160305. [DOI] [PubMed] [Google Scholar]
- Györkey F., Sinkovics J. G., Györkey P. Electron microscopic observations on structures resembling myxovirus in human sarcomas. Cancer. 1971 Jun;27(6):1449–1454. doi: 10.1002/1097-0142(197106)27:6<1449::AID-CNCR2820270627>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györkey F., Sinkovics J. G., Min K. W., Györkey P. A morphologic study on the occurrence and distribution of structures resembling viral nucleocapsids in collagen diseases. Am J Med. 1972 Aug;53(2):148–158. doi: 10.1016/0002-9343(72)90125-8. [DOI] [PubMed] [Google Scholar]
- Huhn D. Neue Organelle im peripheren Lymphozyten? Dtsch Med Wochenschr. 1968 Nov 1;93(44):2099–2100. doi: 10.1055/s-0028-1110887. [DOI] [PubMed] [Google Scholar]
- Imamura M., Muroya K. Lymph node ultrastructure in Hand-Schüller-Christian disease. Cancer. 1971 Apr;27(4):956–964. doi: 10.1002/1097-0142(197104)27:4<956::aid-cncr2820270431>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- JOSEPH R. R., ZARAFONETIS C. J. FATAL SYSTEMIC LUPUS ERYTHEMATOSUS IN IDENTICAL TWINS: CASE REPORTS AND REVIEW OF THE LITERATURE. Am J Med Sci. 1965 Feb;249:190–199. doi: 10.1097/00000441-196502000-00008. [DOI] [PubMed] [Google Scholar]
- Jensen A. B., Spjut H. J., Smith M. N., Rapp F. Intracellular branched tubular structures in osteosarcoma. An ultrastructural and serological study. Cancer. 1971 Jun;27(6):1440–1448. doi: 10.1002/1097-0142(197106)27:6<1440::aid-cncr2820270626>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Jorke D. Die submikroskopische Struktur einer neuen zytoplasmatischen Organelle in Lymphozyten und Lymphoidzellen. Folia Haematol Int Mag Klin Morphol Blutforsch. 1970;94(1):1–10. [PubMed] [Google Scholar]
- Landry M., Winkelmann R. K. Tubular cytoplasmic inclusion in dermatomyositis. Mayo Clin Proc. 1972 Jul;47(7):479–492. [PubMed] [Google Scholar]
- Levy J. A. Autoimmunity and neoplasia. The possible role of C-type viruses. Am J Clin Pathol. 1974 Aug;62(2):258–280. doi: 10.1093/ajcp/62.2.258. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Tannenberg W., Smith C., Schwartz R. S. C-type viruses in systemic lupus erythematosus. Nature. 1974 Nov 1;252(5478):78–79. doi: 10.1038/252078a0. [DOI] [PubMed] [Google Scholar]
- Lockshin M. D., Eisenhauer A. C., Kohn R., Weksler M., Block S., Mushlin S. B. Cell-mediated immunity in rheumatic diseases. II. Mitogen responses in RA, SLE, and other illnesses: correlation with T- and B-lymphocyte populations. Arthritis Rheum. 1975 May-Jun;18(3):245–250. doi: 10.1002/art.1780180308. [DOI] [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieland N. W., Hashimoto K., Masi A. T. Microtubular inclusions in normal skin of systemic lupus erythematosus patients. Arthritis Rheum. 1972 Mar-Apr;15(2):193–200. doi: 10.1002/art.1780150210. [DOI] [PubMed] [Google Scholar]
- Norton W. L. Endothelial inclusions in active lesions of systemic lupus erythematosus. J Lab Clin Med. 1969 Sep;74(3):369–379. [PubMed] [Google Scholar]
- POLLAK V. E. ANTINUCLEAR ANTIBODIES IN FAMILIES OF PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS. N Engl J Med. 1964 Jul 23;271:165–171. doi: 10.1056/NEJM196407232710401. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Pincus T., Blacklow N. R., Grimley P. M., Bellanti J. A. Glomerular microtubules of systemic lupus erythematosus. Lancet. 1970 Nov 21;2(7682):1058–1061. doi: 10.1016/s0140-6736(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Pothier L., Uzman B. G., Kasac M. M., Saito H., Adams R. A. Immunoglobulin synthesis and tubular arrays in the endoplasmic reticulum in transplanted human tumors of lymphoid origin. Lab Invest. 1973 Dec;29(6):607–613. [PubMed] [Google Scholar]
- Quan S. G., Chi E. Y., Caplin S. M. Tubular structures in endoplasmic reticulum of cultured broccoli. J Ultrastruct Res. 1974 Jul;48(1):92–101. doi: 10.1016/s0022-5320(74)80047-x. [DOI] [PubMed] [Google Scholar]
- Schaff Z., Barry D. W., Grimley P. M. Cytochemistry of tubuloreticular structures in lymphocytes from patients with systemic lupus erythematosus and in cultured human lymphoid cells: comparison to a paramyxovirus. Lab Invest. 1973 Dec;29(6):577–586. [PubMed] [Google Scholar]
- Schaff Z., Heine U., Dalton A. J. Ultramorphological and ultracytochemical studies on tubuloreticular structures in lymphoid cells. Cancer Res. 1972 Dec;32(12):2696–2706. [PubMed] [Google Scholar]
- Schumacher H. R., Jr Tubular paramyxovirus-like structures in synovial vascular endothelium. Ann Rheum Dis. 1970 Jul;29(4):445–447. doi: 10.1136/ard.29.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzman B. G., Saito H., Kasac M. Tubular arrays in the endoplasmic reticulum in human tumor cells. Lab Invest. 1971 Jun;24(6):492–498. [PubMed] [Google Scholar]
- White J. G. Lymphocyte inclusions. Ann Intern Med. 1972 Jun;76(6):1042–1043. doi: 10.7326/0003-4819-76-6-1042. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Strand M., August J. T. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974 Oct 1;140(4):1011–1027. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The ultrastructure of lymphocytes. Semin Hematol. 1969 Jan;6(1):4–27. [PubMed] [Google Scholar]